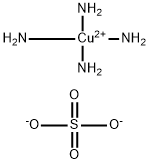
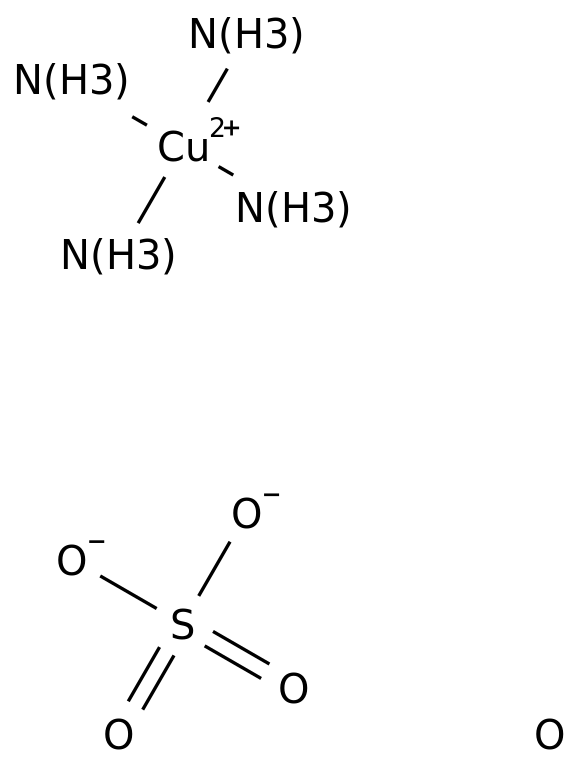
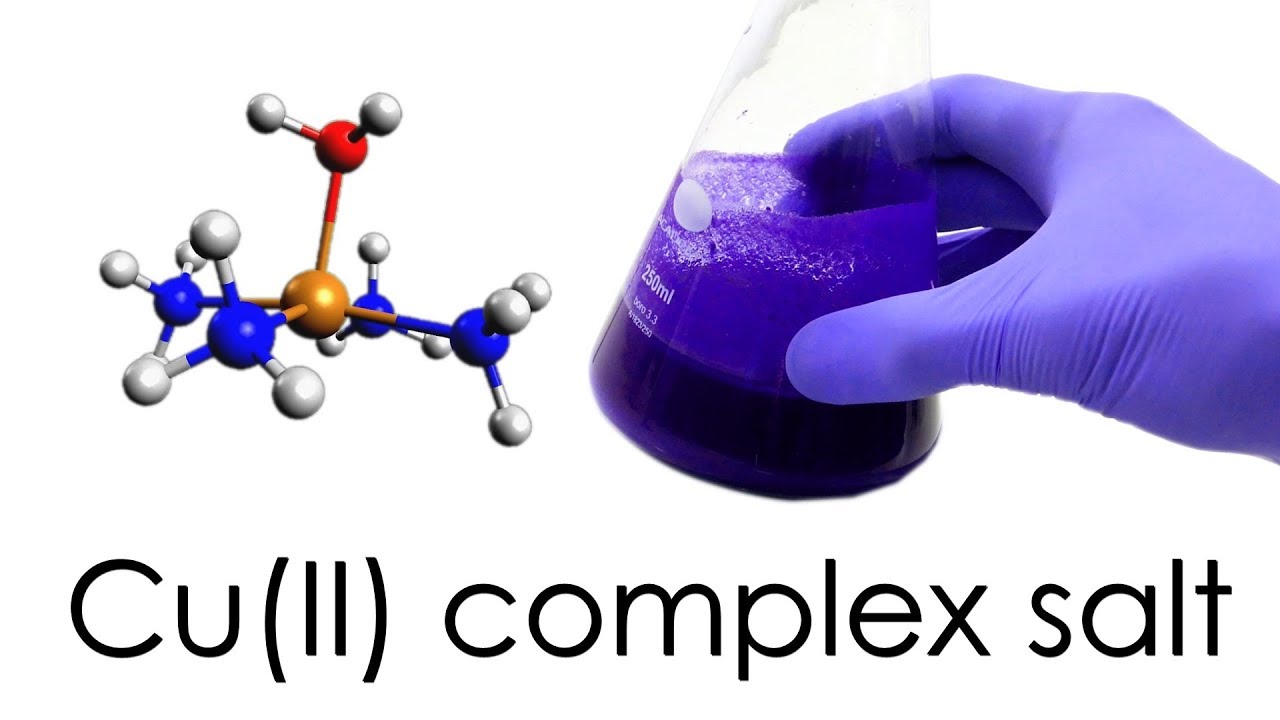
Tetraamminecopper(II), also known as copper(II) ammonia complex or copper ammonia, is an inorganic compound that is made up of copper, ammonia, and water. It is a coordination compound, which means that it is made up of a metal ion bonded to a group of molecules or ions called ligands. In this case, the metal ion is copper(II), and the ligands are four ammonia molecules.
Copper(II) ammonia complex is a deep blue or violet color and is often used as a pigment in paints, inks, and dyes. It is also used as a catalyst in the production of plastics, resins, and rubber. Copper(II) ammonia complex is synthesized by dissolving copper(II) sulfate in a solution of ammonia and water. The resulting solution is blue in color due to the presence of the copper(II) ammonia complex.
Copper(II) ammonia complex is highly soluble in water and is often used in water treatment plants to remove impurities from drinking water. It is also used in the production of fertilizers, as it can help to increase the growth rate of plants.
Copper(II) ammonia complex is a stable compound, but it can decompose if it is exposed to strong acids or bases. It can also be converted back to its original components if it is heated to high temperatures.
Overall, tetraamminecopper(II) is an important compound with a variety of applications in industries such as textiles, plastics, and water treatment. It is highly soluble in water and has the ability to act as a catalyst in various chemical reactions, making it an essential compound in many industrial processes.