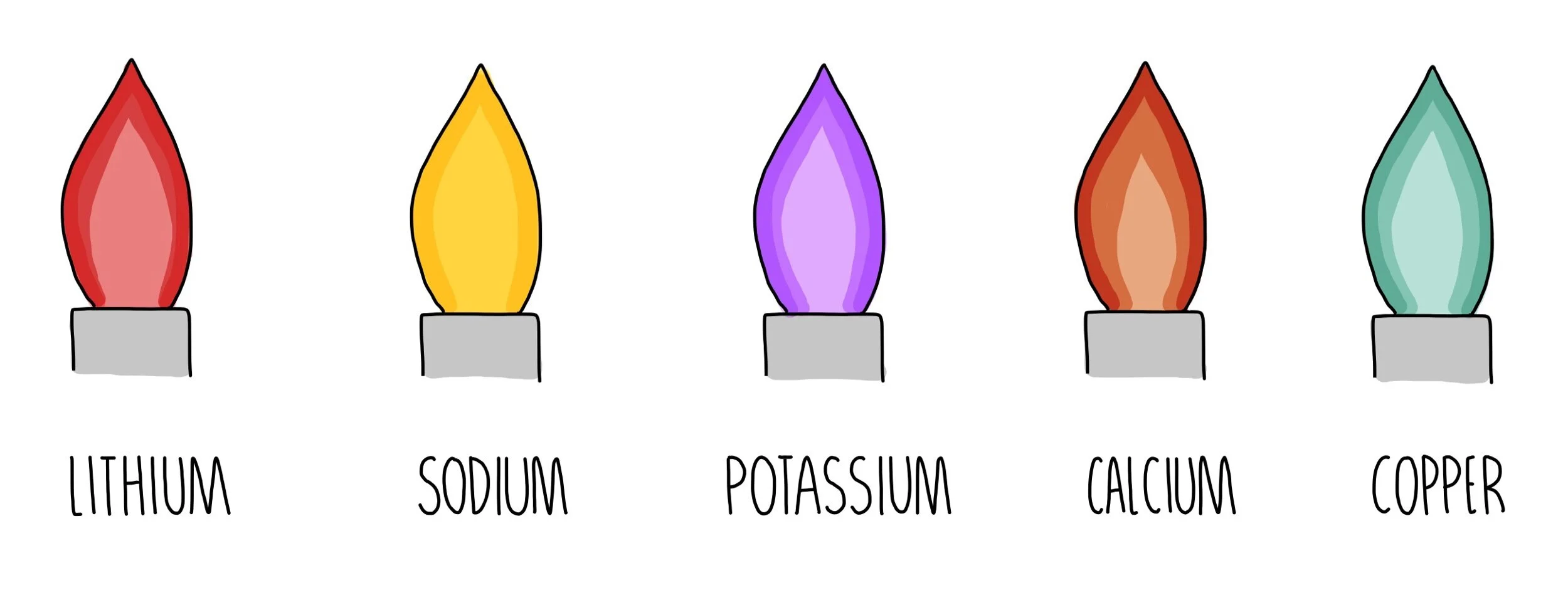
A flame test is a common laboratory technique used to identify the presence of certain elements in a sample by observing the colors produced when the sample is heated in a flame. One element that can be identified through a flame test is potassium, which is a silvery-white metal that is highly reactive and found in a variety of compounds, including potassium chloride (KCl).
To conduct a flame test for potassium chloride, a small amount of the sample is placed on a platinum or nichrome wire, which is then introduced into a flame. As the sample is heated, the electrons in the atoms of the element become excited and release energy in the form of light. The specific wavelengths of light emitted by the element are characteristic of that element and can be used to identify it.
In the case of potassium chloride, the flame test will produce a lilac or violet color. This color is produced by the emission of light at wavelengths of around 766 nanometers, which falls within the visible light spectrum and is perceived as violet by the human eye.
It is important to note that other elements can also produce a violet color in a flame test, so the presence of this color does not necessarily indicate the presence of potassium chloride. Instead, the flame test should be used in conjunction with other techniques, such as spectroscopy, to confirm the identity of the element in question.
Overall, the flame test is a simple but effective tool for identifying the presence of certain elements, including potassium chloride. By observing the colors produced when a sample is heated in a flame, scientists can gain valuable insights into the composition of a substance and its potential uses.
Potassium chloride flame test?

In presence of chlorine, CuCl is formed, emitting strongly in blue. Main emission lines of Strontium: Copper In the flame test, the copper salts give the flame a green-blue color that is between 500 and 550 nm. Sodium, present in sweat, is present in most compounds and will mask the actual colour of the flame. Proceedings of the Physical Society. How to Do the Flame Test? Similarly, when sodium chloride is subjected to a bunsen burner, some sodium ions regain their electrons to form neutral sodium atoms. Why do different atoms give off different colors of light? Later, splints are removed from the distilled water and rinsed.
Potassium Chloride (KCl)

Other analytical procedures should be conducted in addition to this test. Il fenomeno del quenching smorzamento si verifica quando alcune molecole assorbono l'energia dei fluorofori. The solutions can be retained for future use. Lilac Flame tests for metal ions Ion present Flame test colour Lithium, Li + Crimson Sodium, Na + Yellow Potassium, K + Lilac Calcium, Ca 2+ Orange-red What color does potassium chloride make fire? PURPLE flame Fabulous Fun Facts: How to Turn Fire Different Colors Chemical Flame Change Potassium Chloride water softener salt PURPLE flame Copper Chloride BLUE flame Borax laundry LIGHT GREEN flame Copper Sulfate tree root killer for plumbers GREEN flame What color does sodium chloride turn a flame? Do not rub eyes as all chlorides are irritant, corrosive, toxic or harmful. This energy corresponds to particular wavelengths of light, and the colour of its flame reveals the hiding element. Neveruse spray bottles with a rubber bulb - the flame may flash back into the container. When performing the flame test, it should be free from contamination.
Flame Test: How to Identify Metal Ions in a Compound
The brightness of the coloured flame varies from one sample to another. Why is hydrochloric acid used in the flame test? Assuming your burner flame is blue, it may be difficult to see a big color change. Cotton Swabs Cotton swab is another way to do the flame test. Lilac Flame tests Ion present Flame test colour Potassium, K + Lilac Calcium, Ca 2+ Orange-red Barium, Ba 2+ Green Copper, Cu 2+ Blue-green Why do certain elements produce color when heated in a flame? On returning to their original energy level, they emit energy that can be seen in the form of colors by the naked eye. Then it is placed on the blue flame of a bunsen burner. Â Medicinal Use of Potassium Chloride In the human body, potassium is essential for several vital functions to occur, the most notable of which is the beating of the heart. Retrieved 2 May 2013.