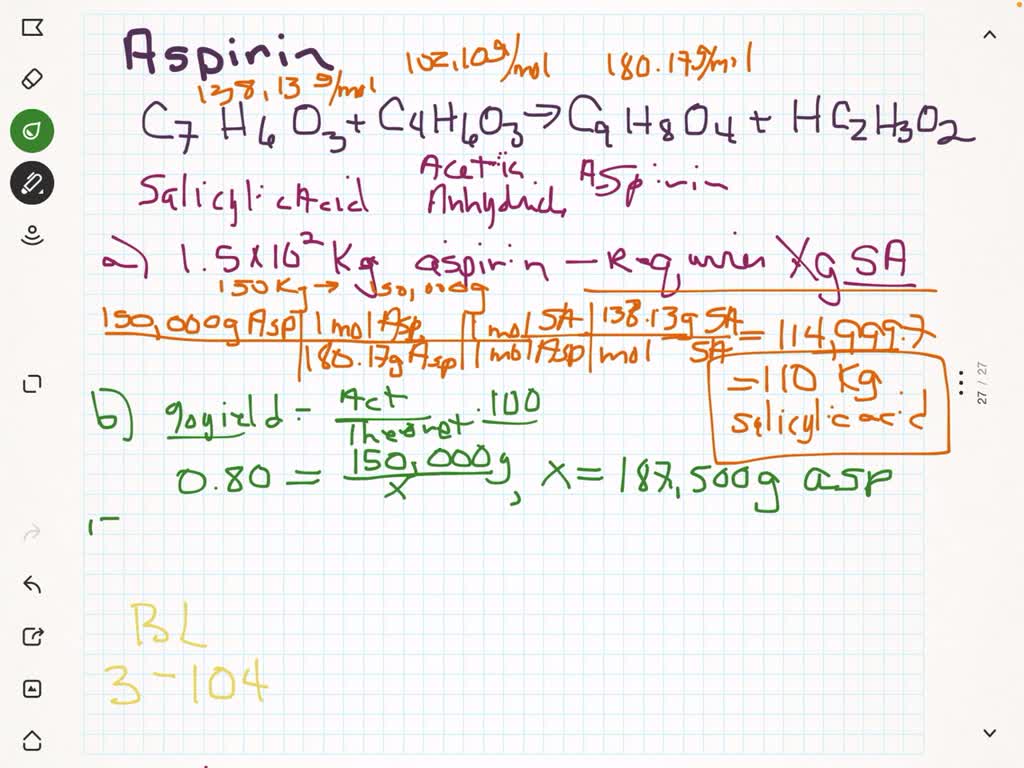Aspirin, also known as acetylsalicylic acid, is a common over-the-counter pain reliever and anti-inflammatory medication. The percent yield of a chemical reaction refers to the amount of product that is actually obtained compared to the theoretical yield, which is the maximum amount of product that could be obtained based on the stoichiometry of the reaction. Calculating the percent yield of aspirin can help chemists understand the efficiency of the synthesis reaction and identify any potential issues that may have occurred during the synthesis process.
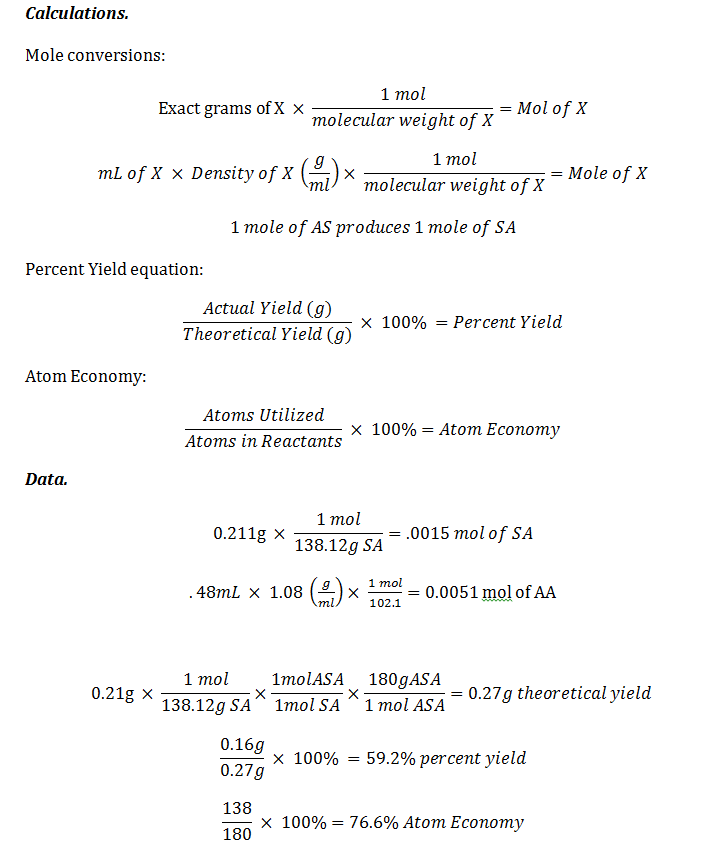
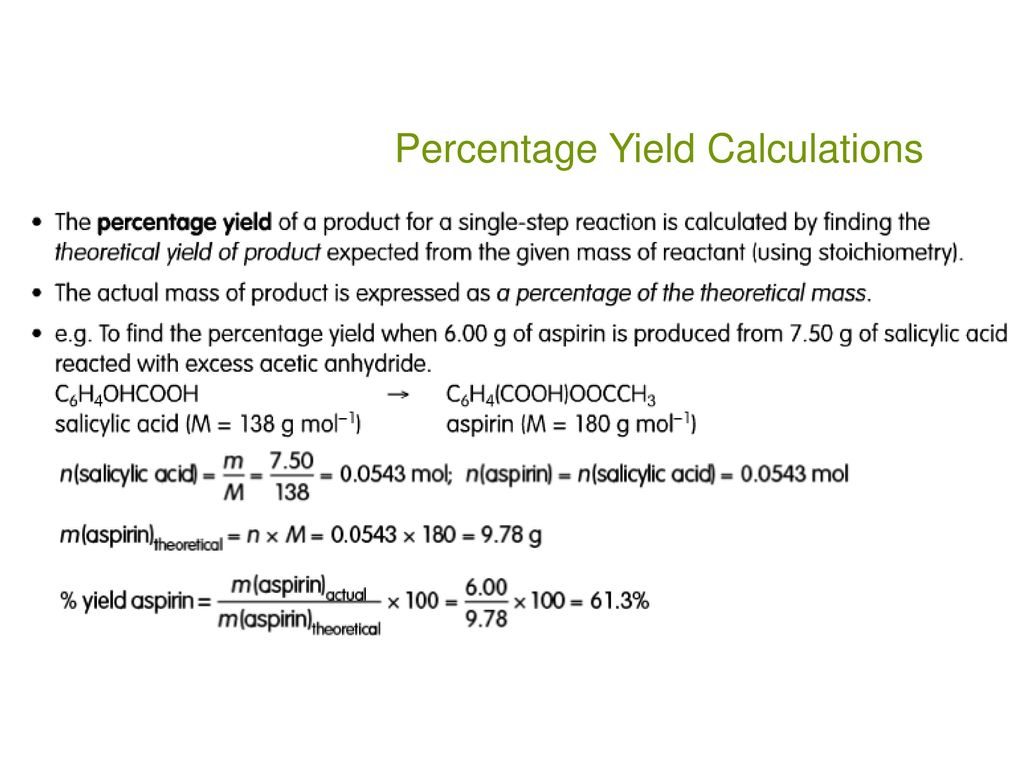
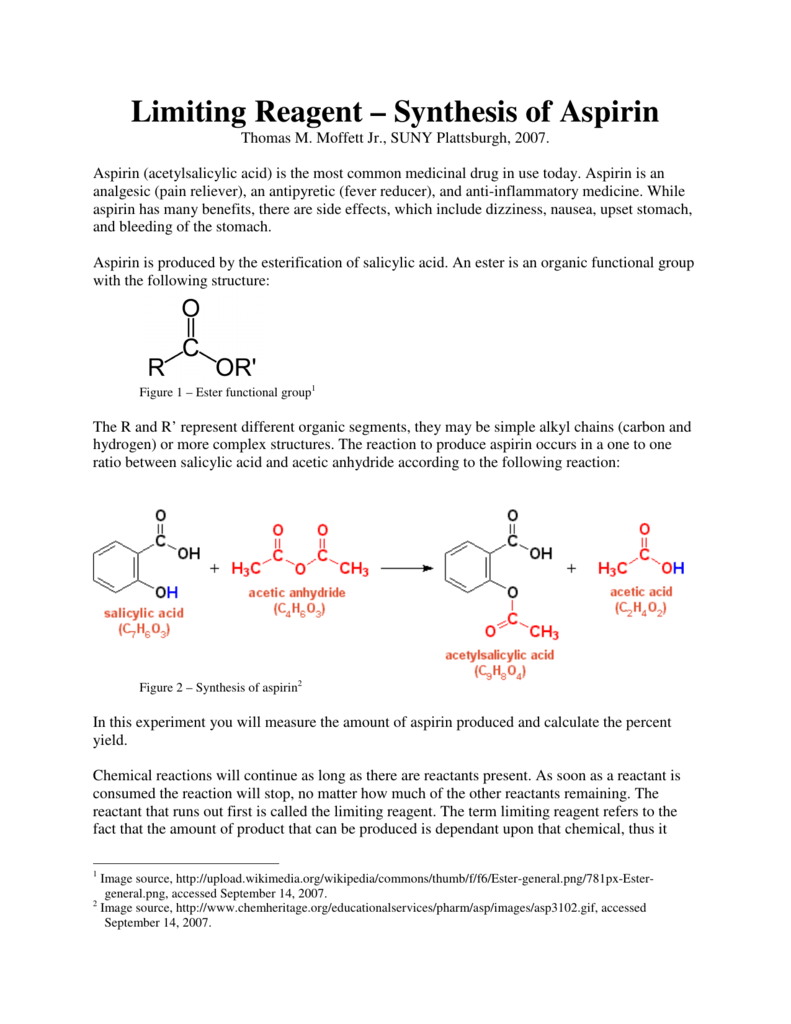
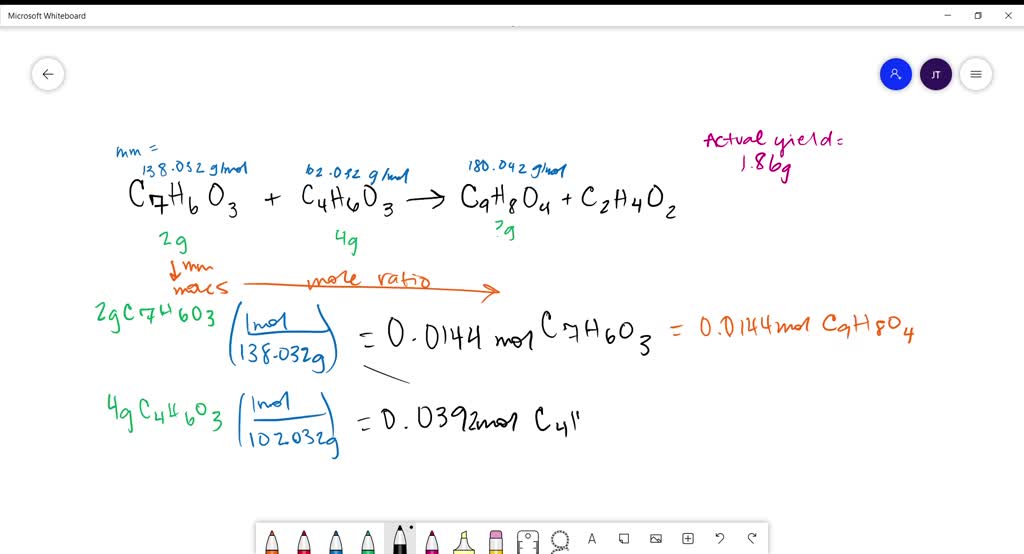
To calculate the percent yield of aspirin, a chemist would need to first determine the theoretical yield of the reaction. This can be done by using the balanced chemical equation for the synthesis of aspirin, which involves the reaction of salicylic acid with acetic anhydride. The balanced equation for this reaction is:
C7H6O3 + C4H6O3 -> C9H8O4 + CH3COOH
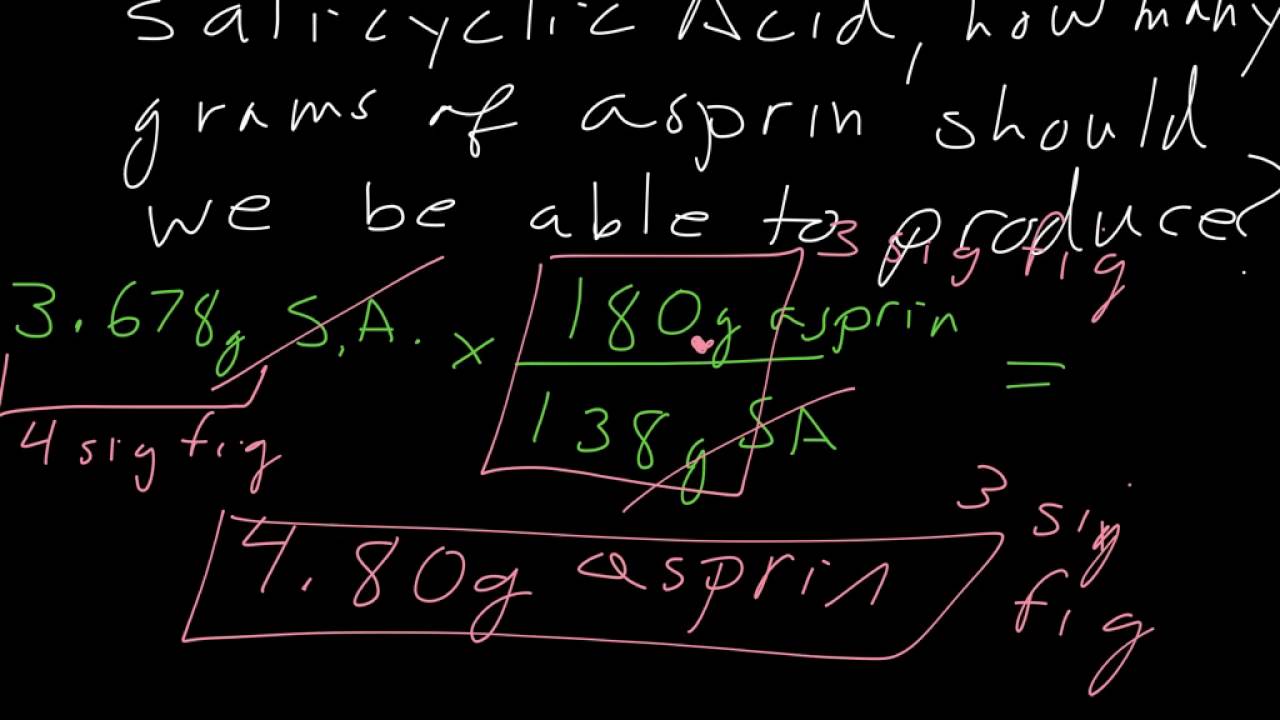
From this equation, it is clear that for every mole of salicylic acid and acetic anhydride that are used in the reaction, one mole of aspirin and one mole of acetic acid will be produced. To determine the theoretical yield of aspirin, the chemist would need to know the starting amounts of salicylic acid and acetic anhydride, as well as the molecular weight of aspirin.
Once the theoretical yield of aspirin has been determined, the chemist would then need to determine the actual yield of the synthesis reaction. This can be done by weighing the amount of aspirin that was obtained after the synthesis reaction has been completed. The actual yield of aspirin can then be expressed as a percentage of the theoretical yield by dividing the actual yield by the theoretical yield and multiplying by 100%.
For example, if a chemist conducted a synthesis reaction using 1 gram of salicylic acid and 1 gram of acetic anhydride, and obtained 0.9 grams of aspirin, the percent yield of the synthesis would be 90%. This indicates that the synthesis reaction was relatively efficient, as 90% of the theoretical yield of aspirin was obtained.
It is important to note that there are many factors that can affect the percent yield of a chemical reaction, including the purity of the starting materials, the efficiency of the reaction conditions, and the presence of any contaminants or impurities. By carefully controlling these factors and accurately measuring the starting materials and products, chemists can optimize the percent yield of a chemical reaction and maximize the efficiency of the synthesis process.