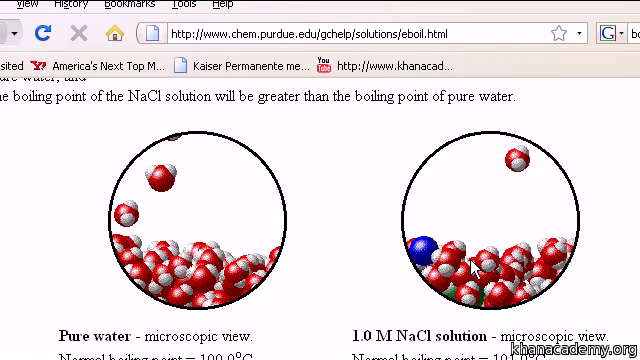
Salt has a significant effect on the boiling point of water. When salt is added to water, it increases the water's boiling point. This is because the salt ions interact with the water molecules, disrupting the balance of forces that holds them together.
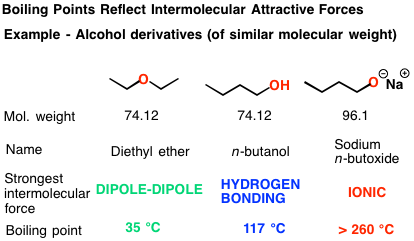
At the molecular level, water molecules are attracted to each other by a type of chemical bond called a hydrogen bond. These bonds are relatively weak, but they are important for maintaining the structure of water molecules. When water boils, the heat energy causes the water molecules to vibrate more and more rapidly, breaking these bonds and allowing the molecules to escape into the air as steam.
However, when salt is added to water, it introduces additional ions into the mixture. These ions are charged particles, and they can interact with the water molecules through an electrostatic force called the Coulomb force. This force acts to increase the attractive forces between the water molecules, making it more difficult for them to break free and boil.
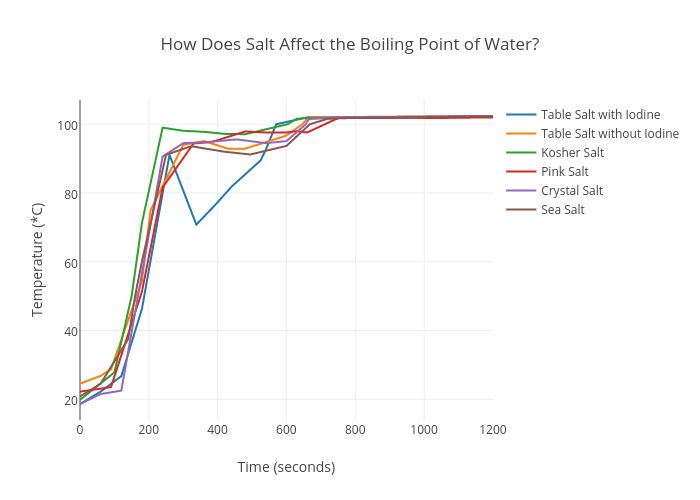
As a result, the boiling point of salt water is higher than the boiling point of pure water. The exact increase in boiling point depends on the concentration of salt in the water. The more salt that is added, the higher the boiling point will be.
For example, if you add a small amount of salt to a pot of water, the boiling point might only increase by a few degrees. However, if you add a large amount of salt, the boiling point could increase by several degrees. This increase in boiling point can have practical implications in certain situations, such as when cooking food that requires a specific temperature to cook properly.
In summary, salt affects the boiling point of water by increasing the attractive forces between water molecules, making it more difficult for them to break free and boil. The exact increase in boiling point depends on the concentration of salt in the water.