The Most Dangerous Game, written by Richard Connell, is a thrilling short story about a hunter named Sanger Rainsford who becomes the prey in a twisted hunting game organized by a wealthy Russian aristocrat named General Zaroff.
The story begins with Rainsford and his friend, Whitney, discussing their differing beliefs about the value of hunting. Rainsford, an experienced hunter, believes that hunting is the ultimate sport because it requires skill and strategy, while Whitney sees it as a barbaric and unnecessary activity.
As the story progresses, Rainsford finds himself stranded on an island after falling overboard from his yacht. He is eventually discovered by General Zaroff, who invites him to stay at his mansion on the island. However, Rainsford soon learns that Zaroff has a twisted hobby: he hunts humans as the ultimate prey.
Zaroff tells Rainsford that he has grown bored of hunting animals and has turned to hunting humans because they provide a more challenging and exciting hunt. He offers Rainsford the chance to join him in the hunt, but Rainsford refuses and becomes the target instead.
The story reaches its climax as Rainsford uses his skills as a hunter to outwit Zaroff and turn the tables on him. In the end, Rainsford emerges as the victor, having proven himself to be the better hunter.
The Most Dangerous Game is a thrilling and suspenseful story that explores the themes of survival, the value of human life, and the dark side of human nature. It highlights the dangerous consequences of allowing one's desires and ego to override moral principles and shows the power of intelligence and resourcefulness in overcoming adversity.
Overall, The Most Dangerous Game is a thought-provoking and entertaining tale that serves as a cautionary warning about the dangers of indulging in dangerous and unethical pursuits.
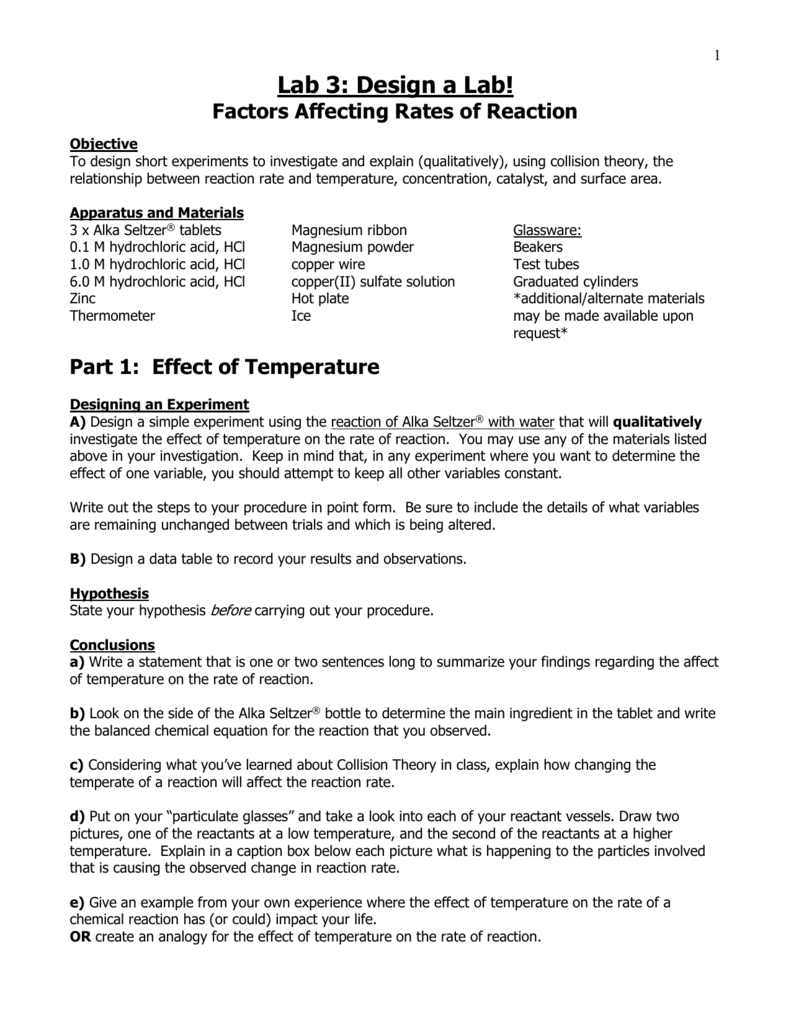
Factors Affecting Reaction Rate Lab Report Assignment free sample

Conclusion After performing experiments on how different factors affect reaction rate. Is it possible to vary the factors because, in terms of concentration, if we left water in a test tube and we add acid to it, the acidity is going to lower, and it will not be strong enough to react with the metal, and the collision will have already happened with the acid and H2O. We controlled the amount of hydrogen peroxide and the amount of each catalysts to make sure that the change of compounds are the only factor that affect the rate of decomposition. Repeat step 2 for the other test tube. If it took more than 18 — 22 seconds, repeat the steps 1 — 10 but now using 1 less drop of Na 2 S 2 O 3 solution 13.
Factors Affecting Reaction Rates webapi.bu.edu

The slowest reaction rate was the 1 co. This is because more surface area was exposed and more of the CaCO3 was able to react with the hydrochloric acid than the marble chips. Results: Particle Size Substance Tested Observations CaCO3 Reacted quickly; more surface area is exposed Marble chips Reacted quickly as well, but not as fast. Because particles have to collide to react successfully, so an increase in concentration of produces makes more collisions. For Part 3, we predicted that the more surface area the reactants have, the higher the rate of the chemical reaction will be. In part 1, temperature was a hard variable to control well, the temperature of two trials might not be accurately same which might affected the result of the experiment.
Factors Affecting Reaction Rates

By doubling the rate, yes you are doubling the reaction rate, because larger kinetic energy, more collisions and if you look at the temperature graph above, o can see the steep increase of the graph. This hypothesis was made according to the information we found in the textbook: Increasing the temperature of the reactants can cause the particles to move more quickly. This experiment will identify the rate constant from 5 solutions utilizing the above equation, only adding different concentrations of buffer, potassium iodide, starch, sodium thiosulfate, and hydrogen peroxide. Additionally by performing essentially the same experiments but with temperature changes one can determine how k is affected by temperature changes and the new activation energy. Watch the video on moodle to answer HCl , H 2 SO 4 , CH 3 COOH, H 3 PO 4 -List the metals in order of decreasing reaction rate with 6M HCl. The stock solutions are the following: 4 M acetone, 1 M HCl, and 0 M iodine.