Testing a leaf for starch is a common experiment in biology classrooms, as it allows students to understand the process of photosynthesis and how plants use energy. In this lab report, we will outline the materials and methods used, describe the results of the experiment, and discuss the implications of these results.
Materials:
- Fresh leaf from a green plant
- Iodine solution
- Beaker
- Test tube
- Glass stirring rod
- Dropper
- Paper towels
Methods:
- Obtain a fresh leaf from a green plant and gently wash it with water to remove any dirt or debris.
- Fill a beaker with water and add a few drops of iodine solution.
- Use a dropper to place a small drop of the iodine solution onto the leaf.
- Observe the color of the iodine on the leaf. If the leaf contains starch, the iodine will turn blue or black. If the leaf does not contain starch, the iodine will remain yellow or orange.
- Repeat the process with a few additional drops of iodine to confirm the results.
- If necessary, use a glass stirring rod to scrape a small piece of tissue from the leaf and place it in a test tube. Add a few drops of iodine solution to the test tube and observe the color change.
Results:
In our experiment, we found that the iodine turned blue or black when applied to the leaf, indicating the presence of starch. When a small piece of tissue was placed in a test tube and mixed with iodine solution, the solution also turned blue or black. These results suggest that the leaf we tested contains starch.
Discussion:
Starch is a complex carbohydrate that plants use to store energy. It is produced during photosynthesis, when the plant uses energy from sunlight to convert carbon dioxide and water into glucose. The glucose is then converted into starch and stored in the plant's tissues, such as leaves, stems, and roots.
The presence of starch in the leaf we tested confirms that the plant is able to carry out photosynthesis and produce glucose. This is important for the plant's survival, as it allows the plant to store energy for times when sunlight is not available, such as at night or during periods of low light intensity.
Overall, testing a leaf for starch is a simple and effective way to understand the process of photosynthesis and the role of starch in plant metabolism. It also helps students learn how to use scientific equipment and follow experimental procedures, which are important skills for any aspiring scientist.
CIS

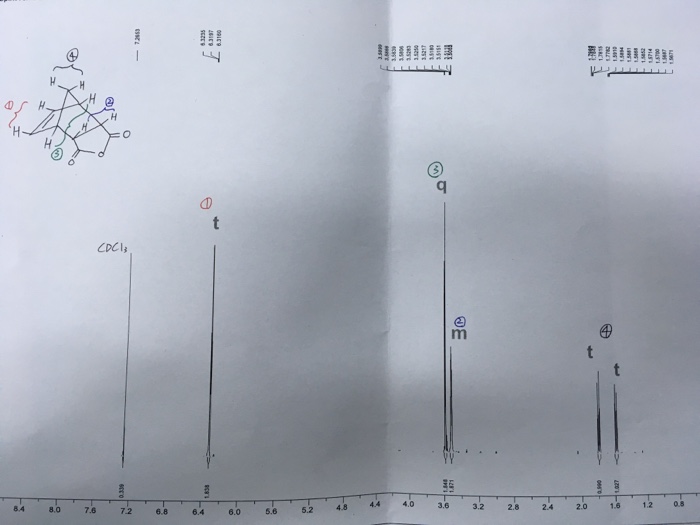
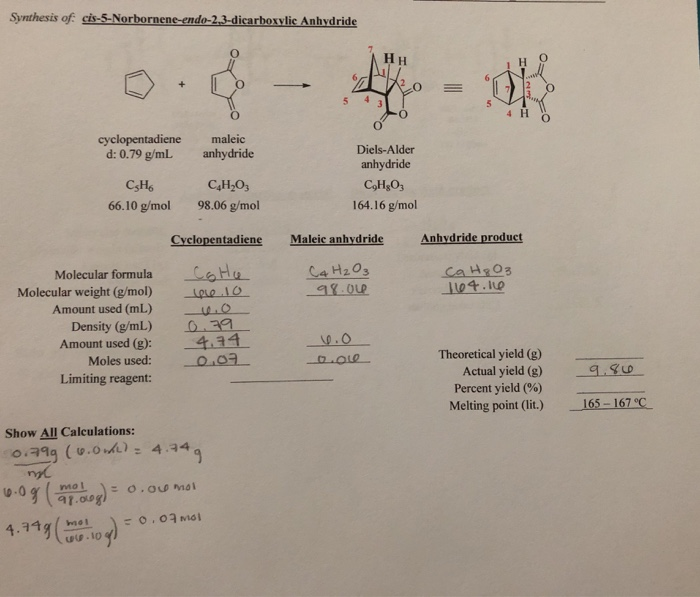
Next 2 mL of acetic acid was added to the test tube and the solution turned a cloudy white color. In order to un- dimerize dicyclopentadiene, it must be heated to just under its boiling point to make fresh cyclopentadiene. Reference Table for compounds used in Diel-Alder lab. Throughout the experiment, the mixture changed color from green, orange, to yellowish lime, and eventually clear. Also, the percent yield. The calculations for the percent yield are as follows: First, the moles of the reactants must be calculated: Through these calculations we can see that the cyclopentadiene is the limiting reagent is the cyclopentadiene because the reaction between cyclopentadiene and maleic anhydride is a 1:1 reaction.
cis
GHS H Statement H334-H318-H317 May cause allergy or asthma symptoms or breathing difficulties if inhaled. It has been in studies of ribozymes and creating ribozymes synthetically Jaschke, 2000. Even though our crystals did not turn out as we had hoped, the melting point range for our crystals was very close to the literature value. The chemicals used are Maleic anhydride and Cyclopentadiene. In a future experiment, this reaction needs to be performed very slowly and with a lot of precision to minimize contamination in the final product. Therefore, the sodium borohydride reduction of the ketone, 9-fluorenone was performed to yield the secondary alcohol, 9-fluorenol. Add additional ice if needed but do not allow to get in flask.
Synthesis of Cis
The identity of the product and unknown were 4-tert-butylbenzyl phenol ether and tert-butyl phenol respectively. This low yield can be explained from a poor recrystallization technique combined with potential contamination. At the end of the experiment, to prove the formation of the major products, melting point of the products were measured. Reduction of an organic molecule usually corresponds to decreasing its oxygen content or increasing its hydrogen content. Synthesis of Diphenylacetylene Observation of Results: 1,2-dibromo-1,2-diphenylmethane 0. The reaction process lasted for 2 hours. Melting point of the product was also determined to verify the correct product was obtained and to check the purity of the product.

We were able to form cis-Norbornene-5, 6-endo-dicarboxylic anhydride crystals and were able to achieve a very pure product. Thus, the substance that is still in the product made it have a lower melting point than it should have. Initially, the color of the reaction turned into a dark green color and over time became a lighter shade with a minimal solid left. The Diels- Alder reaction is one of the most important reactions in all of organic chemistry because of the applicability of it. The methanol acts as a solvent for the reaction as a nucleophile. In the case of cyclopentadiene and maleic anhydride, the reaction takes place quite quickly due to the many electronegative oxygen present in both reactants.

In this experiment, the melting point and of cis-Norbornene-5,6-endo-Dicarboxylic anhydride is determine. This type of reaction was named for Otto Diels and Kurt Alder who were the first to investigate this reaction Weldegirma, 2012. Diels- Alder reactions are used constantly in the medical field due to its diverse uses. The triphenylmethanol looked like a white powder. Bromination Lab Report 785 Words 4 Pages In this experiment, it was possible to produce the major products from bromination of acetanilide and aniline. Conclusion: The Diels- Alder reaction is one of the most important types of reactions in organic chemistry Weldegirma, 2012.