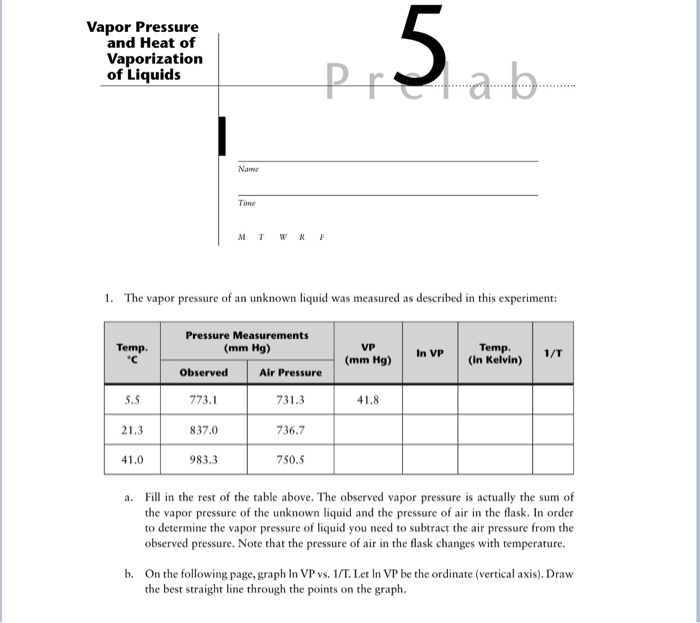
Vapor pressure and heat of vaporization are important physical properties of a substance that can be measured in a laboratory setting. Vapor pressure is defined as the pressure exerted by the vapor of a substance in equilibrium with its liquid or solid form at a given temperature. Heat of vaporization, on the other hand, is the amount of heat required to convert a given amount of a substance from a liquid to a gas at a constant temperature. In this essay, we will discuss a lab experiment that measures these properties and the principles behind them.
The most common method for measuring vapor pressure is the Clausius-Clapeyron equation, which relates the vapor pressure of a substance to its temperature and the heat of vaporization. This equation can be used to determine the vapor pressure of a substance at a given temperature by measuring the heat of vaporization and the temperature at which the vaporization occurs.
To measure the heat of vaporization and vapor pressure of a substance in a lab setting, a setup called a vapor pressure apparatus can be used. This apparatus consists of a container filled with the substance in question, a thermometer to measure the temperature, and a manometer to measure the pressure. The substance is heated until it reaches its boiling point, at which point the vapor pressure becomes equal to the atmospheric pressure. The temperature and pressure are then recorded as the substance continues to boil.
As the substance continues to boil, the heat of vaporization can be calculated by measuring the heat added to the substance over time. This is done by measuring the change in temperature of a known mass of water surrounding the substance as it boils. The heat of vaporization can then be calculated by dividing the change in temperature of the water by the mass of the water.
In addition to the Clausius-Clapeyron equation and the vapor pressure apparatus, there are other methods for measuring vapor pressure and heat of vaporization. One such method is the Knudsen effusion method, which involves measuring the rate at which a gas effuses through a small hole. The heat of vaporization can also be measured using the bomb calorimeter, which measures the heat of a reaction in a closed system.
In conclusion, vapor pressure and heat of vaporization are important physical properties that can be measured in a laboratory setting using a variety of methods. Understanding these properties can provide valuable information about the behavior of a substance and its potential uses.