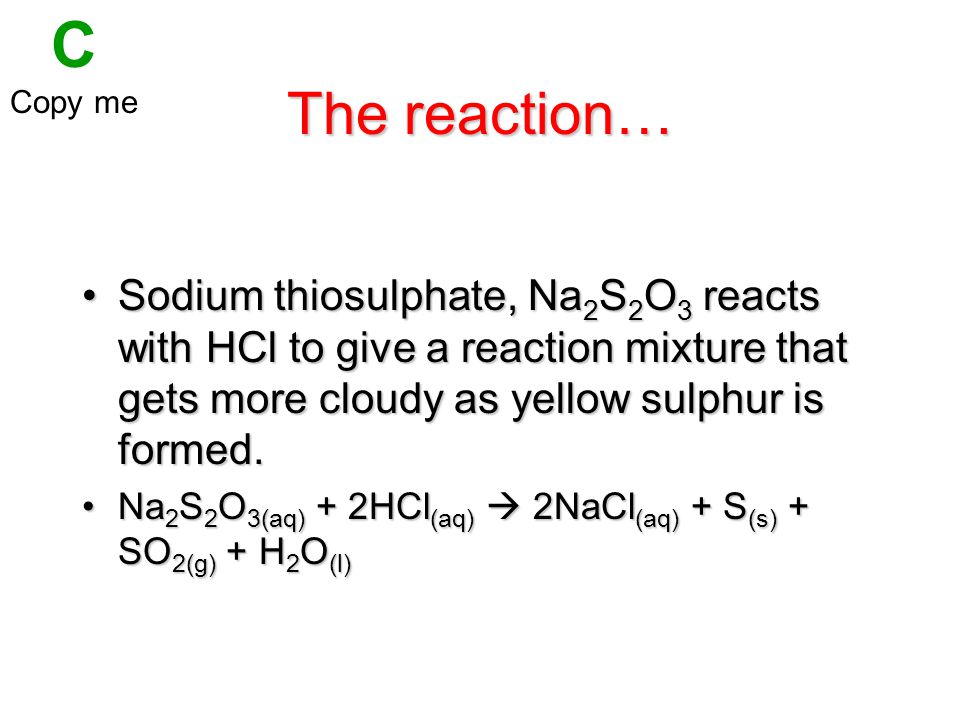
Thiosulfate (S2O3^2-) and hydrochloric acid (HCl) are two chemicals that can react together to form a number of different products, depending on the conditions of the reaction. In this essay, we will explore the different ways that thiosulfate and hydrochloric acid can interact, and consider the applications and significance of these reactions.
One common reaction between thiosulfate and hydrochloric acid is the formation of sulfur dioxide (SO2) and sulfuric acid (H2SO4). This reaction can be represented by the following balanced chemical equation:
S2O3^2- + 2HCl -> SO2 + 2H+ + Cl^- + S
This reaction is exothermic, meaning that it releases heat when it occurs. The sulfur dioxide produced in this reaction can be used in a variety of industrial processes, such as the production of sulfuric acid and the purification of natural gas.
Another reaction that can occur between thiosulfate and hydrochloric acid is the formation of tetrathionate (S4O6^2-), a polysulfur anion with a structure similar to that of thiosulfate. This reaction can be represented by the following balanced chemical equation:
2S2O3^2- + 6HCl -> 2S4O6^2- + 6H+ + 3Cl^-
Tetrathionate is an intermediate compound in the biological process known as sulfur oxidation, which is important for the removal of sulfur compounds from the environment. It is also used in the laboratory as a reagent for the determination of sulfur in chemical samples.
In addition to these two reactions, thiosulfate and hydrochloric acid can also form other products, such as sulfate (SO4^2-), hydrogen thiosulfate (H2S2O3), and sulfuric acid (H2SO4). The specific products that are formed will depend on the conditions of the reaction, including the concentration of the reactants and the presence of other substances that may influence the reaction.
Overall, the reactions between thiosulfate and hydrochloric acid are important for a variety of industrial and scientific applications. They can be used to produce valuable chemicals, such as sulfur dioxide and tetrathionate, and to study the behavior of these compounds under different conditions. Understanding the reactions between thiosulfate and hydrochloric acid can also help us to better understand the role of these compounds in natural and biological systems.