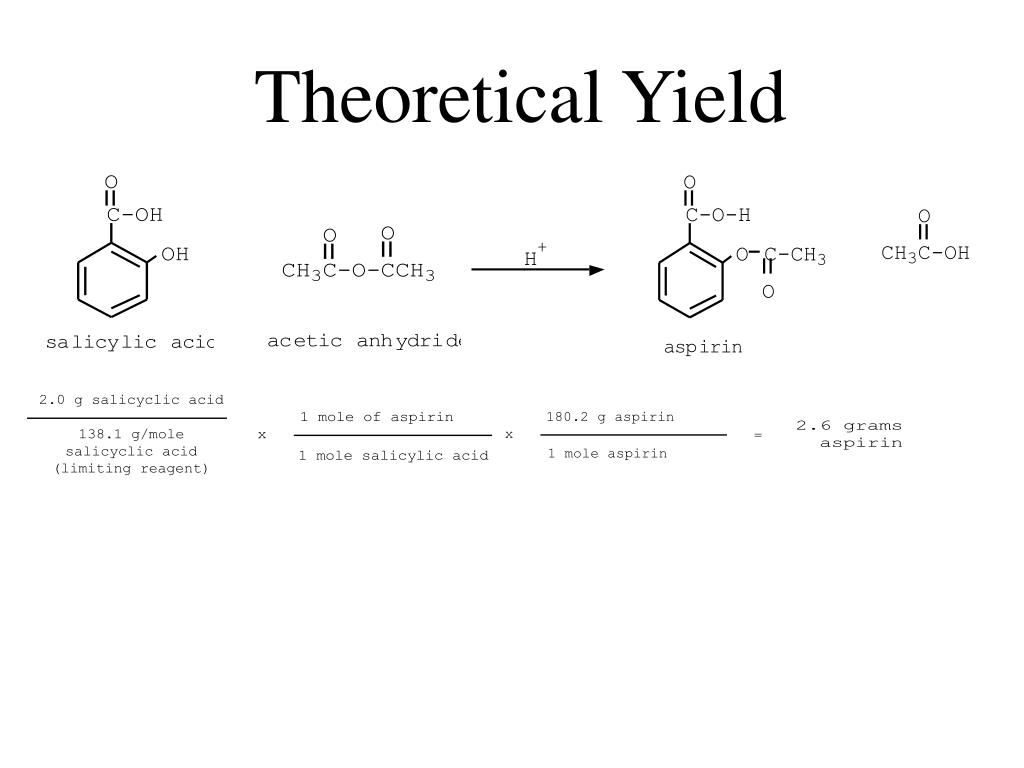
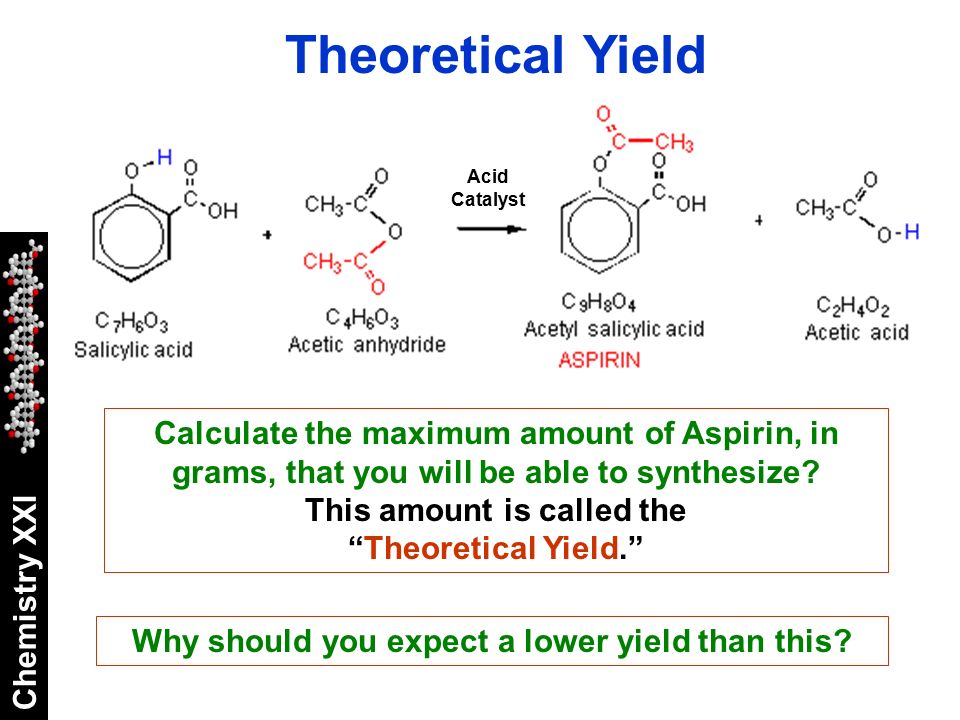
Aspirin, also known as acetylsalicylic acid, is a widely used non-steroidal anti-inflammatory drug (NSAID) that is used to relieve pain, reduce inflammation, and lower the risk of heart attack and stroke. The synthesis of aspirin involves the conversion of salicylic acid into acetylsalicylic acid through the reaction with acetic anhydride. This reaction is known as acetylation and is typically carried out in the presence of an acid catalyst, such as sulfuric acid.
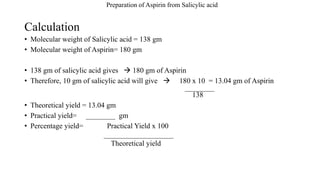
The theoretical yield of aspirin synthesis is the maximum amount of aspirin that can be produced from a given amount of reactants. It is calculated based on the stoichiometry of the reaction, which is the ratio of reactants and products in a chemical reaction. In the case of aspirin synthesis, the stoichiometry is 1 mol of salicylic acid reacting with 1 mol of acetic anhydride to produce 1 mol of acetylsalicylic acid and 1 mol of acetic acid.
The theoretical yield can be calculated by using the following equation:
Theoretical yield = (Reactant mass / Reactant molar mass) x (Product molar mass / Product molar mass)
For example, if we start with 100 grams of salicylic acid and 50 grams of acetic anhydride, the theoretical yield of aspirin can be calculated as follows:
Theoretical yield = (100 g / 138.12 g/mol) x (180.16 g/mol / 180.16 g/mol) = 72.3 g
This means that the maximum amount of aspirin that can be produced from these reactants is 72.3 grams.
However, it is important to note that the actual yield of aspirin synthesis may be less than the theoretical yield due to various factors such as incomplete reactions, side reactions, and losses during purification. These factors can affect the efficiency of the synthesis and reduce the amount of aspirin produced.
In conclusion, the theoretical yield of aspirin synthesis is the maximum amount of aspirin that can be produced from a given amount of reactants based on the stoichiometry of the reaction. The actual yield may be lower due to various factors that can affect the efficiency of the synthesis.