Aspirin, also known as acetylsalicylic acid, is a commonly used pain reliever and anti-inflammatory medication. It is a white, crystalline powder that is soluble in water and has a melting point of around 135-137°C. The melting point of a substance is the temperature at which it changes from a solid to a liquid.
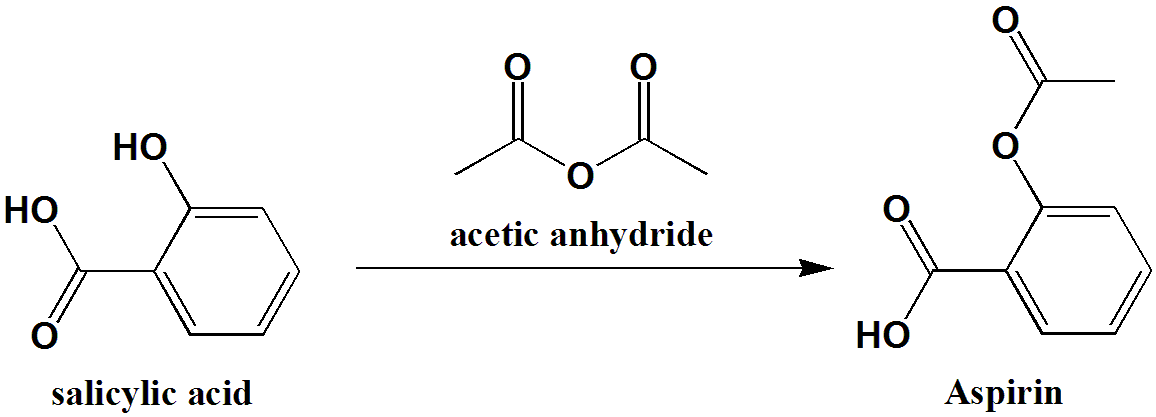
Aspirin is synthesized from salicylic acid and acetic anhydride, and its melting point is determined by the strength of the intermolecular forces within the compound. These forces, known as van der Waals forces, are attractive forces between the molecules of a substance that help to hold them together. The stronger the van der Waals forces, the higher the melting point of the substance.
The melting point of aspirin is relatively low compared to other common medications, such as paracetamol, which has a melting point of around 168°C. This means that aspirin is more prone to melting or decomposing at higher temperatures, which can affect its stability and effectiveness. Therefore, it is important to store aspirin in a cool, dry place to help ensure its potency and shelf life.
In addition to its use as a pain reliever, aspirin has several other important medical applications. It is used to reduce the risk of heart attacks and stroke by preventing the formation of blood clots, and it can also be used to treat fever and inflammation. Aspirin is available in various forms, including tablets, capsules, and chewable tablets, and is widely available over the counter at pharmacies and other retail outlets.
In conclusion, the melting point of aspirin is around 135-137°C, and it is an important factor in determining the stability and effectiveness of the medication. While it has a relatively low melting point compared to other substances, it is still widely used and has numerous medical applications.
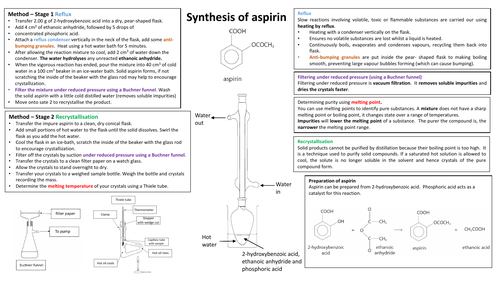
The Chemistry of Aspirin
National Heart Foundation of Australia. The insoluble portion was gravity filtered and air dried to yield 0. Later, the compound salicylic acid named for the Latin word for willow, salix was isolated from willow bark; it proved to be the active ingredient. Also, when transferring the crude aspirin into the vacuum filtration, some of the crystals stayed in the flask. Aspirin, therefore, has an analgesic reduces pain , anti-inflammatory reduces redness and swelling , anti-platelet reduces blood clots and antipyretic temperature reduction effects 1,2,3. When heating the salicylic acid mixture in the warm water bath, the mixture should be removed from the bath within 8 minutes, to reduce the chance of the acetylsalicylic acid decomposing. He is also the designate chairman of the Cardiovascular Disease Branch of Chinese Medical Association, council member of the Cardiovascular Angiography and Interventions Association, international consultant of the American Heart Association.
Aspirin

The Reverend Edward Stone, a vicar from Chipping Norton, Oxfordshire, England, noted in 1763 that the bark of the willow was effective in reducing a fever. When adding a few drops of concentrated sulfuric acid, which acted as a catalyst, effervescence transpired and some of the salicylic acid dissolved. The American Journal of Medicine. The purpose of this procedure is to isolate acetylsalicylic acid from its accompanied acid catalyst, unreacted salicylic acid, acetic anhydride, and other impurities. Aspirin is also believed to help reduce the risk of heart attacks. The Journal of Clinical Investigation.
Aspirin Melting Point Analysis

Displays anticancer effects in some solid tumors. It is known to have a sour taste due to the acid that is present in the tablets. Aspirin is prepared by chemical synthesis from salicylic acid, through acetylation with acetic anhydride. New England Journal of Medicine. The known melting point range for acetyl salicylic acid is 135°C -136°C. In cancer, aspirin is believed to impact a number of cancer signalling pathways and may induce or upregulate cancer suppressor genes 3. It has the ability to reduce fever an antipyretic , to reduce pain an analgesic , and to reduce swelling, soreness, and redness an anti-inflammatory agent.