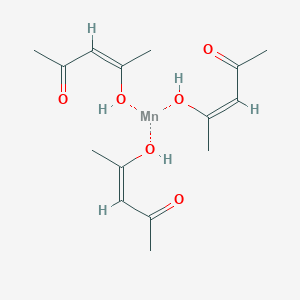
The synthesis of manganese acetylacetonate (Mn(acac)3) involves the reaction of manganese oxide with acetylacetone. The balanced equation for this synthesis is:
2 MnO + 4 acacH -> 2 Mn(acac)3 + 2 H2O
Manganese oxide is a compound that can be found in nature as the mineral pyrolusite. It is typically prepared through the reduction of manganese dioxide, which is obtained from natural sources or synthesized through the electrolysis of manganese sulfate. Acetylacetone, also known as 2,4-pentanedione, is a colorless liquid that has a sweet, fruity odor. It is a common reagent used in the synthesis of various compounds, including Mn(acac)3.
In the synthesis of Mn(acac)3, manganese oxide and acetylacetone are mixed together in a reaction vessel. The mixture is then heated to a high temperature, typically around 200°C, to facilitate the reaction. As the mixture is heated, the acetylacetone reacts with the manganese oxide to form Mn(acac)3 and water as the final products. The water is produced as a byproduct of the reaction and is typically removed through distillation.
Mn(acac)3 is a yellow, crystalline solid that is commonly used as a precursor to other manganese compounds. It has a variety of applications, including the synthesis of pigments, catalysts, and magnetic materials. It is also used as a starting material in the synthesis of other manganese compounds, such as manganese dioxide and manganese sulfate.
Overall, the synthesis of Mn(acac)3 is a relatively simple process that involves the reaction of manganese oxide with acetylacetone. The resulting compound has a wide range of applications and is an important intermediate in the synthesis of other manganese compounds.
Metal Acetylacetonate Synthesis

After each addition, firmly tap the tube on the hard surface. To minimize this problem, the sample should be finely powdered sue a mortar and pestle and introduced into the tube in small portions. After the addition is complete, boil the solution for 5 minutes, and then chill the beaker in ice. What happens when you oxidize a ketone with permanganate? I also need the reaction mechanism for this reaction. The materials were massed once before and once after being heated in the drying oven. With the field on, weigh the tube again.
12: The Paramagnetic Complex Mn(acac)3 (Experiment)

To prevent this from happening, the hot plate should not exceed 130˚C, so no matter what ketone was isolated, it would not evaporated off. When the solid is completely dissolved, record the NMP spectrum of the solution see your instructor for directions concerning the use of the NME instrument. Please complete any questions as much as you can before posting. What are acac complexes used for? Fill the tube to the line with water at the existing temperature, the volume V may be calculated. If you add the acetyl acetone too quickly, the solution will generate large amounts of foam that may overflow the flask. Mz+ + z acacH M acac z + z H+ Complexes consist of a central metal atom surrounded by various other atoms or small molecules called ligands.
webapi.bu.edu

Four unpaired electrons exist in the high spin complex, which makes it paramagnetic, while no unpaired electrons exist in the low spin complex, which is diamagnetic, and hence, a low spin configuration is adopted by the cobalt complex. Compounds that contain a coordination complex are called coordination compounds. Complexes can be positively charged, neutral or negatively charged. The Viton and Silver Shield gloves are not disposable. To remove paramagnetic impurities from the tube, clean it with Nochromix cleaning solution do not use chromic acid, which will add paramagnetic impurities! This was accomplished with a Diels-Alder reaction that utilized 3-nitrobenzaldehyde, acetophenone, ethanol, and sodium hydroxide.

The melting point of the product from the bromination of acetanilide was 164. A special NMR tube is needed to carry out this measurement. Some of the acidic compound may have entered the basic solution and reacted with the base to form a high melting point salt, making the melting point of the base appear abnormally high. Into the NMR tube, place a sealed capillary that contains pure chloroform, CHCl 3 the capillaries that can be made by syringing CHCl 3 into a melting point capillary, and then sealing the open end in a flame. Please ignore Report question 1 and Problems 2, 5 and 10. It is prepared by the direct reaction of acetylacetone and potassium permanganate. I looked up in the literature and there was no explicit redox equation for the reaction.







3]+–+Magnetic+Susceptibility.jpg)