Medea is a tragic play written by the ancient Greek playwright Euripides, which tells the story of a woman named Medea who takes revenge on her ex-husband, Jason, and his new wife by killing their children. The play is set in Corinth, a city in ancient Greece, and it explores themes of love, loyalty, and betrayal.
Medea is a complex and multifaceted character who is driven by her deep love for her children and her desire to protect them at all costs. She is also motivated by a sense of betrayal and anger towards Jason, who has abandoned her and their children in order to marry a wealthy princess. Medea is torn between her love for her children and her desire for revenge, and ultimately chooses to take the latter path, killing her children in order to hurt Jason and his new wife.
The play explores the theme of love and its consequences, as Medea's love for her children ultimately leads her to commit a horrific act. It also delves into the theme of loyalty, as Medea must choose between her loyalty to her children and her loyalty to her husband. The play ultimately suggests that loyalty and love can sometimes be at odds with one another, and that the choices we make in the name of love can have devastating consequences.
The play also touches upon the theme of betrayal, as Jason betrays Medea by leaving her and their children for another woman. Medea's reaction to this betrayal is extreme, but it is a clear expression of the depth of her love for her children and her desire to protect them.
In conclusion, Medea is a tragic play that explores themes of love, loyalty, and betrayal. It tells the story of a woman who is torn between her love for her children and her desire for revenge, and ultimately chooses to take a violent and tragic path. The play serves as a cautionary tale about the dangers of love and the consequences of our actions.
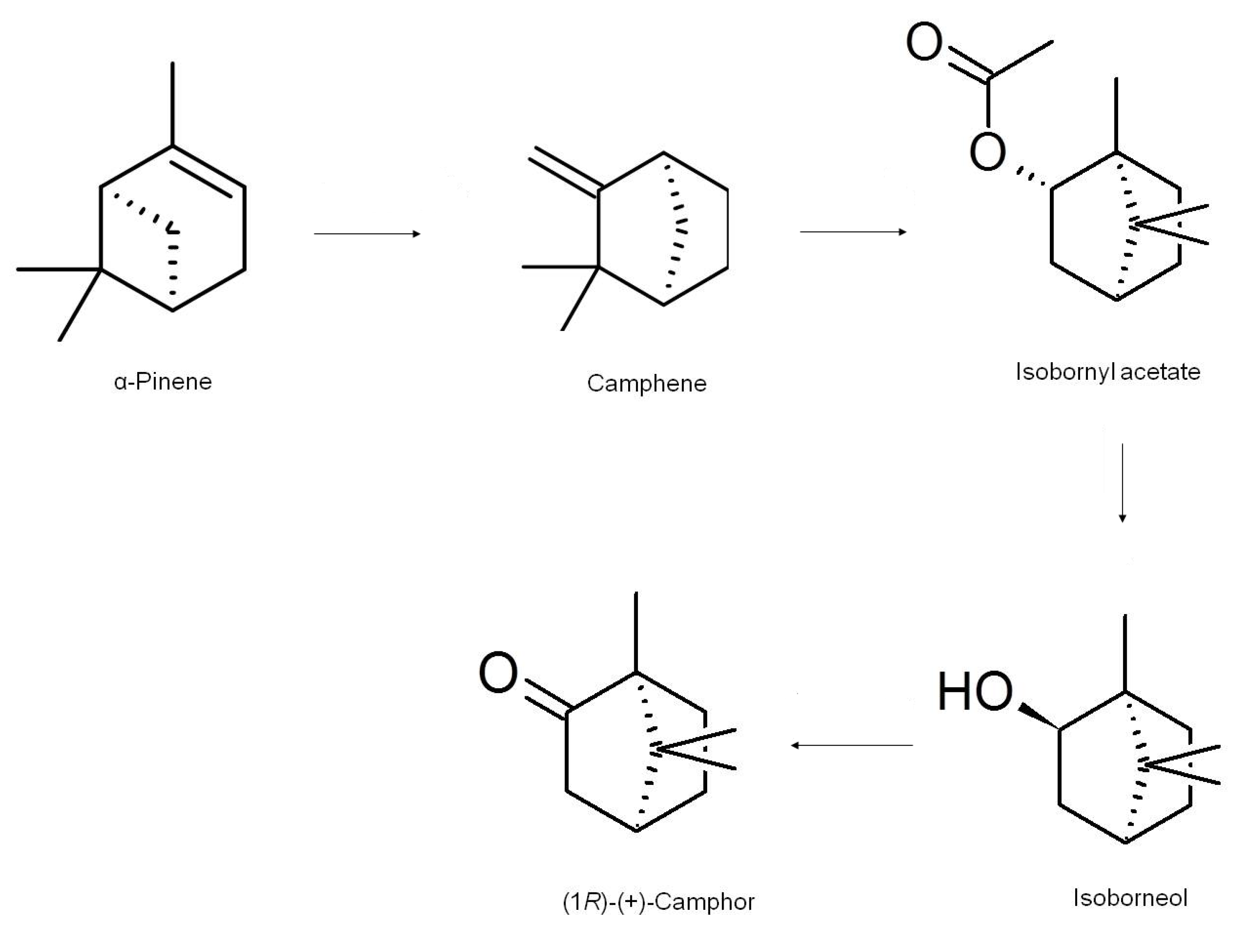
[PDF] Synthesis Of Camphor By The Oxidation Of Borneol

Oxidation of Borneol to Camphor Using Oxone and Catalytic Sodium Chloride: A Green Experiment for the Undergraduate Organic Chemistry Laboratory. In the second stage, compared with sample 3c, basically it continued the trend of the first stage, and the weight loss was still the lowest, which indicated that the resin might have a certain delay effect on the weight loss of coating system. Assymetric SynthesisThe Reaction of Turpentine with Oxalic Acid, and Its Application to a Camphor SynthesisI. Pacific Affairs 1932, 5, 797-801 3 Green, B. Green Oxidation of 1S -Borneol to 1S -Camphor with Oxone Scheme Mechanism: The green agent used in the experiment, oxone, oxidizes the chloride ion from the NaCl into a molecular chloride. The purpose of he experiment was to efficientlysynthesize camphor in an environmentally friendly way. The attachment area of algae is calculated by image processing software and the data of 10 points are averaged.
Borneol

Standard Practure for the Preparation of Substitute Ocean Water. This procedure was repeated on day 14, 30, 48, 56, and 64. Although camphor can be easily steam-distilled from a specific tree, that tree is not always readily available. Carefully move the container into an illuminating incubator. This experiment successfully produced only environmentally benign waste by only producing excess salt in the aqueous solution, which in the end of lab was neutralized and washed with water down the drain. The sublimation caused white crystal product to be observed on the bottom of a round bottom flask and the inner walls of the beaker of the sublimation apparatus. The content of IBOMA decreased from 50% to 16.
Synthesis

GC, GC-MS,1H NMR, IR, and melting point were all used to determine confirm the efficiency of the synthesis andpurification of camphor by the oxidation of borneol. Each glass slide used was weighted before and after the coating to determine the initial dry weight W 0 of each sample. Full size image Fig. This could be positively changed by involving a reaction at the end to restore the active state of the oxone. Recently, oxone has been used as an oxidizing agent which offers a safe alternative to the commonly used chromium reagents. This indicated that the proportion of TIPSA in the whole system was limited, which was not enough to change the weight loss effect of the whole system. The second green chemistry principle present in the experiment was the use of safer reagents.
Synthesis of Camphor

The oxygenfrom the water molecule then deprotonates borneol and the hydrogen carbon bond electrons are pushedto the oxygen carbon bond to produce a double bond while the chlorine acts as a leaving group. Journal of Physics: Conference Series 2007, 61, 643-646 5 Clark, J. Yield of Crude Product Table 2 Theoretical Actual % Yield Yield grams Yield grams Correct yield grams Corrected yield % Literature Melting Point Celsius Melting Point Celsius 1. All the other two mouths of this bottle connect the hydrogen plenum system respectively and insert thermometer, the outer heating system that is provided with of bottle. A novel XRF method to measure environmental release of copper and zinc from antifouling paints. Although the change of IBOMA content does not significantly affect the release rate of camphor, the decrease of IBOMA content corresponds to the antifouling performance of the resin system.