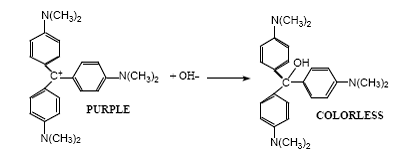
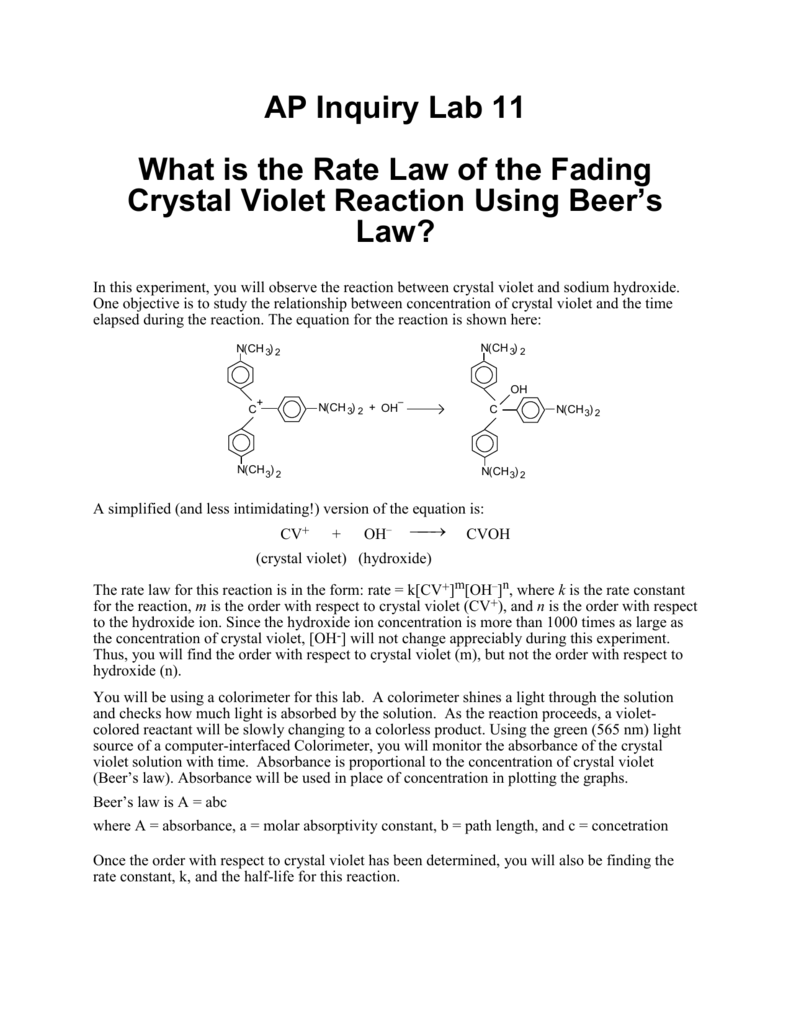
Crystal violet, also known as gentian violet or methyl violet, is a water-soluble dye with a deep purple color. It is commonly used as a biological stain and has a variety of industrial and medical applications. When crystal violet is mixed with a strong base such as sodium hydroxide (NaOH), a chemical reaction occurs that results in the formation of a new compound.
The reaction between crystal violet and NaOH is an example of a neutralization reaction, in which an acid and a base react to form a salt and water. Crystal violet is a weak acid, with a pK a value of about 5.5. This means that it can partially dissociate into its ions in solution, with the proton (H+) being donated to the solvent. On the other hand, NaOH is a strong base, which means that it readily donates its hydroxide ions (OH-) to the solvent.
When crystal violet is mixed with NaOH, the acid and the base react to form the salt sodium crystal violet and water. The reaction can be represented by the following equation:
C 25 H 30 ClN 3 + NaOH -> NaC 25 H 30 ClN 3 + H 2 O
In this reaction, the H+ ions from the crystal violet molecule combine with the OH- ions from the NaOH to form water, while the rest of the crystal violet molecule and the sodium ions combine to form the salt sodium crystal violet.
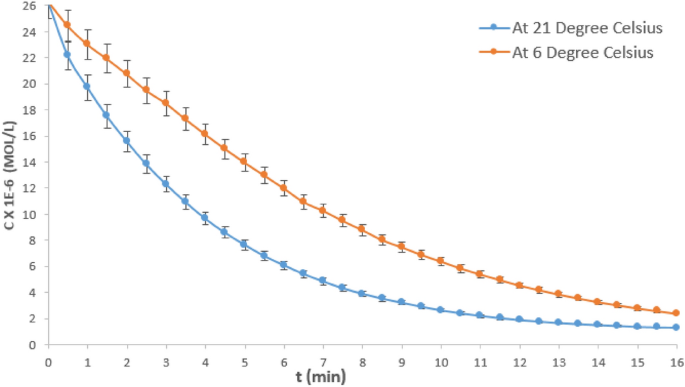
The reaction between crystal violet and NaOH is accompanied by a change in color. Initially, the solution is a deep purple color due to the presence of the crystal violet dye. As the reaction proceeds, the color of the solution changes to a lighter shade of purple or pink, due to the formation of the sodium crystal violet salt. The final color of the solution will depend on the concentration of the reactants and the amount of base used, as well as other factors such as the pH and temperature of the solution.
In conclusion, the reaction between crystal violet and NaOH is a neutralization reaction in which an acid and a base react to form a salt and water. This reaction is accompanied by a change in color, with the solution transitioning from a deep purple color to a lighter shade of purple or pink. Crystal violet is a commonly used dye with a variety of applications, and understanding the chemical reactions it undergoes is important for its various uses.