Quantum numbers are a system of specifying the unique properties of a particular electron in an atom. These numbers are used to describe the energy, angular momentum, and spatial orientation of an electron within an atom. There are four quantum numbers: the principal quantum number (n), the angular momentum quantum number (l), the magnetic quantum number (m_l), and the spin quantum number (m_s).
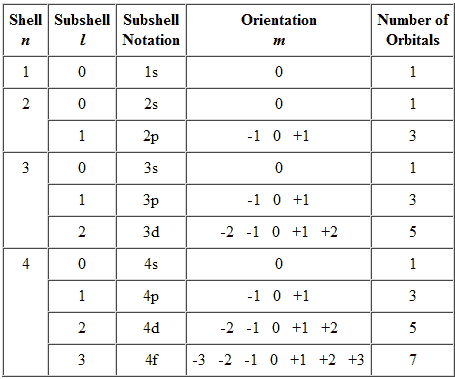
The principal quantum number (n) represents the energy level of the electron. It can take on any positive integer value, and the higher the value, the higher the energy of the electron. For example, an electron with a principal quantum number of 1 is in the lowest energy level (the ground state), while an electron with a principal quantum number of 2 is in the second energy level, and so on.
The angular momentum quantum number (l) describes the shape of the electron's orbital, which is the region of space around the nucleus where the electron is most likely to be found. The value of l can range from 0 to n-1, where n is the principal quantum number. For example, if the principal quantum number is 2 (n=2), then the possible values of l are 0 and 1. Orbitals with l=0 are called s orbitals, while those with l=1 are called p orbitals.
The magnetic quantum number (m_l) describes the orientation of the orbital in space. It can take on any integer value from -l to +l, where l is the angular momentum quantum number. For example, if l=1, then the possible values of m_l are -1, 0, and +1.
The spin quantum number (m_s) describes the spin of the electron, which is a type of angular momentum associated with the electron's motion around its own axis. It can take on only two values: +1/2 or -1/2.
As an example, consider the hydrogen atom, which consists of a single electron orbiting a single proton. The quantum numbers for the electron in the ground state (the lowest energy state) of the hydrogen atom are n=1, l=0, m_l=0, and m_s=+1/2. This means that the electron is in the first energy level, has an s orbital (l=0), is oriented in the xy plane (m_l=0), and has a spin of +1/2.
In summary, quantum numbers are a system of specifying the unique properties of an electron in an atom, including its energy, orbital shape, orbital orientation, and spin. They are important tools in understanding the behavior and properties of electrons in atoms.