The harana is a traditional Philippine string instrument that has a rich history and cultural significance. It is similar to a guitar and is played with a plectrum or pick, using a technique called "tapping."
The harana originated in the Visayan region of the Philippines, where it was traditionally played by men who would serenade women in the evening. This courtship ritual, known as "harana," involved the suitor singing and playing the harana outside the woman's home, hoping to win her affection. The harana and the harana tradition have long been an integral part of Filipino culture and continue to be a popular form of entertainment.
The harana is typically made of wood, with a pear-shaped body and a long neck. It has six strings, which are made of either gut or metal. The strings are plucked or strummed to produce sound, and the player can create a variety of different tones by using different picking techniques.
In modern times, the harana is not only used for courting, but also for performances in both traditional and popular music. It is often featured in Philippine folk music and has also been incorporated into contemporary pop and rock music.
The harana is a unique and beautiful instrument that has a special place in Philippine culture and history. It continues to be enjoyed and appreciated by people of all ages and serves as a reminder of the country's rich musical heritage.
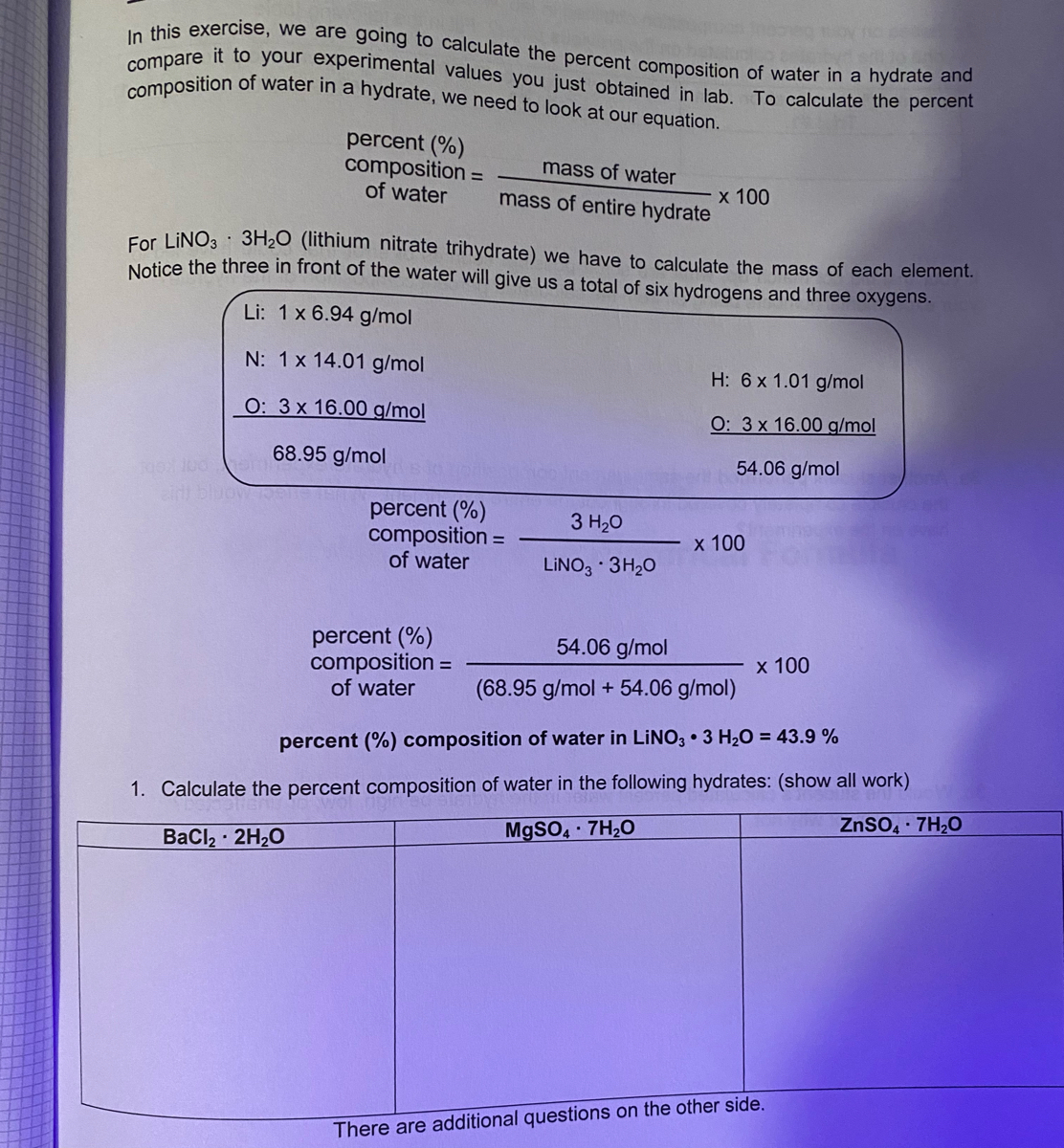
Percent Composition of Hydrates Essay Example
Know the location of the emergency lab shower and eyewash station and the procedures for using them. Otherwise, repeat the process until the mass no longer changes, which indicates that all of the water has evaporated. Due: November 4, 2012 Percent Composition of Hydrates Lab Report Michelle Sims Due: November 4, 2012 Percent Composition of Hydrates Lab Report Michelle Sims Purpose: Demonstrate proficiency in using the balance and Bunsen burner. If you get a chemical in your eyes, immediately flush the chemical out at the eyewash station while calling to your teacher. Know the location of the emergency lab shower and eyewash station and the procedures for using them. Put on safety goggles and lab apron. Make sure equipment is clean, do not touch crucible after it has been heated, and let cool before weighing.
10.11: Percent of Water in a Hydrate

Could the solid be a hydrate? Expelling water from the hydrate. When heating a substance in a test tube, the mouth of the test tube should point away from where you and others are standing. This will help to reduce errors due to small lab balance inaccuracies. Allow the crucible, cover, and contents to cool in the desiccator, and then determine their mass and record it in the data table. Applying conclusions How much water could 25 g of anhydrous CuSO4 absorb? The heat can become so intense that the sulfate in the salt begins to break down. By doing this, a better understanding of hydrates, simple decomposition reactions, and the Law of Definite Determining The Molecular Formula Of A Hydrated Copper Sulfate percentage of water, and the molecular formula, of a hydrated copper II sulfate will be calculated through an understanding of hydrates, percent composition, and moles. Hydrates are solid compounds which contain water.
Percent Composition of Hydrates Lab Manual

Why are hydrates important in chemistry? Drawing conclusions Using your answers from items 3 and 4, determine the empirical formula for the copper sulfate hydrate. Use the glass end to stir the compound. When many ionic compounds are crystallized from a water solution, they include individual water molecules as part of their crystalline structure. Do not taste any chemicals or items used in the laboratory. Call your teacher in the event of an acid spill. If not enough heat is applied, some water will remain attached to the copper sulfate producing a low calculated mass percent water for the hydrate.
Percent Composition and Hydrates Flashcards

The significance of hydration The hydration process has enormous significance for chemical reactions. If an acid or base spills onto your skin or clothing, wash the area immediately with running water. When water is added, copper sulfate turns blue. If you look at a typical bottle of copper sulfate, it will be a bluish-green. Perform calculations by using the molar mass.
2•nH2O+is+heated%2C+and+3.9+g+of+the+anhydrous+salt+remains.+What+is+the+value+of+n.jpg)
You will observe a color change, which is normal, but if the substance remains yellow after cooling, it was overheated and has begun to decompose. The difference between these two masses is equal to the mass of the water lost. The small opening will allow gases to escape. Once you have heated the crucible and cover, do not touch them with your bare hands. Allow the crucible, cover, and contents to cool for 5 min in the desiccator, and then measure their mass. Heat gently for 2-3 minutes and heat to crucible for minutes.







2•nH2O+is+heated%2C+and+3.9+g+of+the+anhydrous+salt+remains.+What+is+the+value+of+n.jpg)
