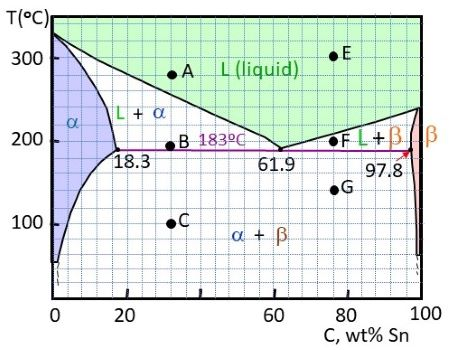
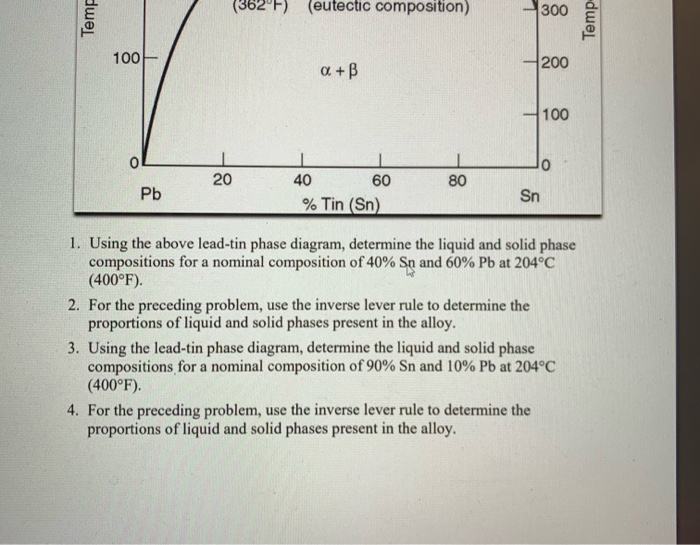
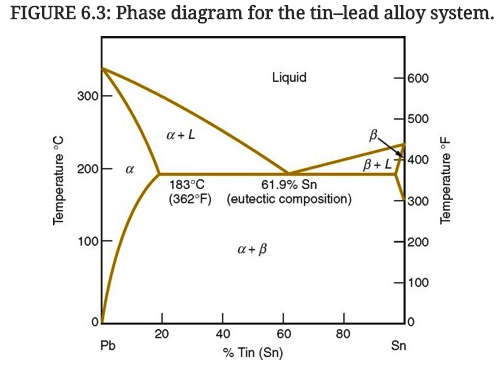
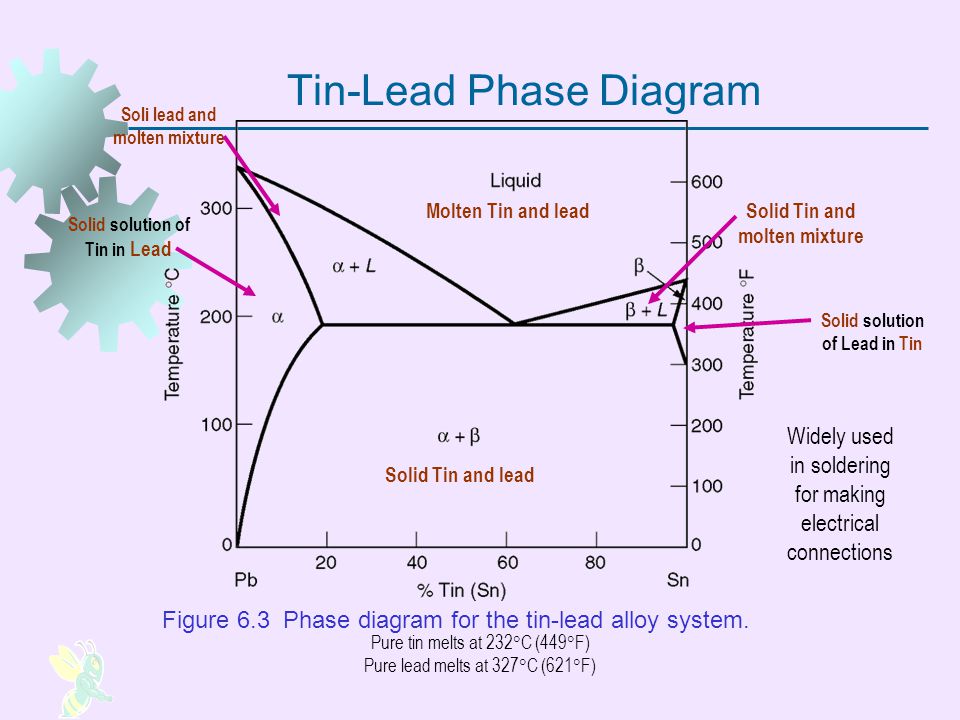
A lead-tin phase diagram is a graphical representation of the different phases or states of a binary alloy system, in this case a mixture of lead and tin, as a function of temperature and composition. It is a useful tool for understanding the behavior of the alloy at different temperatures and compositions, and can be used to predict the microstructure and properties of the alloy.
In a lead-tin phase diagram lab report, the purpose of the experiment is usually to determine the phase diagram for the lead-tin alloy system by performing a series of heating and cooling cycles on samples of the alloy with different compositions. This is typically done using a differential scanning calorimeter (DSC), which measures the heat flow between a sample and a reference material as the temperature is changed.
To begin the experiment, the researcher will first prepare a series of alloy samples with different compositions of lead and tin. These samples can be prepared by melting the two metals together in a crucible and casting the alloy into ingots or discs. The composition of each sample is carefully measured and recorded.
Next, the samples are placed in the DSC and the temperature is gradually increased. As the temperature rises, the alloy will go through various phase transitions, such as the solid-solid transformation from the beta phase to the alpha phase, or the solid-liquid transformation as the alloy melts. The DSC measures the heat flow between the sample and the reference material as these phase transitions occur, and this data is used to construct the phase diagram.
The lead-tin phase diagram is divided into three main regions: the alpha region, the beta region, and the liquid region. The alpha region is the solid state of the alloy at low temperatures, and is characterized by a body-centered cubic (BCC) crystal structure. The beta region is the solid state at higher temperatures, and is characterized by a face-centered cubic (FCC) crystal structure. The liquid region is the molten state of the alloy, in which the atoms are disordered and able to move freely.
The phase diagram also includes several lines or boundaries that mark the transitions between the different phases. The solidus line marks the temperature at which the alloy begins to melt, while the liquidus line marks the temperature at which the alloy is completely melted. The eutectic line marks the composition at which the alloy has the lowest melting point, and the eutectic point marks the temperature at which the eutectic composition is reached.
In a lead-tin phase diagram lab report, the results of the experiment would typically be presented in the form of a graph showing the phase diagram for the lead-tin alloy system. The graph would include the solidus, liquidus, and eutectic lines, as well as the composition and temperature ranges for the alpha, beta, and liquid regions. The report would also include a discussion of the results, including any observations or discrepancies from the expected phase diagram, and any implications for the properties and microstructure of the alloy.
Overall, the lead-tin phase diagram is a valuable tool for understanding the behavior of this alloy system and predicting its microstructure and properties at different temperatures and compositions. By performing a series of heating and cooling cycles using a DSC, researchers can construct the phase diagram and gain valuable insights into the behavior of the alloy.