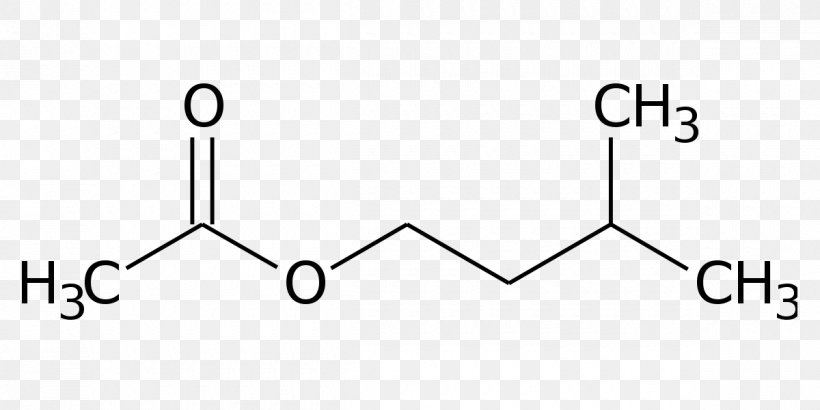
Isopentyl alcohol (also known as isopentanol or 3-methylbutanol) is a clear, colorless liquid with a fruity or sweet smell. It is a common solvent and is often used as a starting material for the synthesis of other chemicals.
When isopentyl alcohol is reacted with acetic acid, it undergoes an esterification reaction to form isopentyl acetate, which is a common flavor and fragrance ingredient. This reaction is typically catalyzed by an acid catalyst, such as sulfuric acid, and proceeds via a nucleophilic acyl substitution mechanism.
Isopentyl acetate is a liquid at room temperature and has a fruity, apple-like aroma. It is used in a variety of consumer products, including perfumes, flavorings, and cleaning products. It is also used as a solvent for paints, resins, and inks.
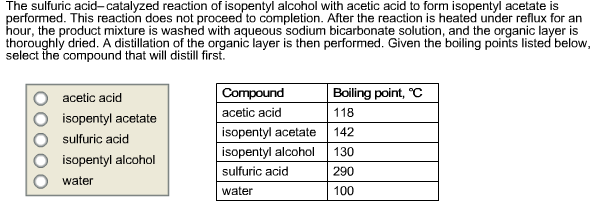
The esterification reaction between isopentyl alcohol and acetic acid is reversible, meaning that it can be reversed under certain conditions. For example, if the reaction mixture is heated to a high temperature, the isopentyl acetate can be hydrolyzed back into isopentyl alcohol and acetic acid.
In summary, isopentyl alcohol and acetic acid can react to form isopentyl acetate through an esterification reaction. Isopentyl acetate is a useful chemical with a fruity aroma that is used in a variety of consumer products. However, the reaction is reversible and can be reversed under certain conditions.
fischer esterification of acetic acid with isopentyl webapi.bu.edu
So we're going to say one mole of cl two produces one mole of c three H five cl. If the yield from the reaction of acetic acid with isopentyl alcohol is 45%, how many grams of isopentyl acetate are formed from 3. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. Record the barometric pressure in the laboratory. What was the "alcohol" used? Gravity filtration was used to further dry the organic layer by running it through a pipette column of anhydrous sodium sulfate. Fusce dui lectus, Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. Nam risus ante, dapibus a molestie consequat, ultrices a tesque dapibus efficitur laoreet.
5: Synthesis of Isopentyl Acetate (Experiment)

The final mass of isopentyl acetate the product was 670 mg, giving a percent 70% yield. Nam lacinia pulvinar tortor nec facilisis. Instead of waiting for a week, like the procedure states, the culture tube was placed in a hot sand bath and massed the same day. If the yield from the reaction of acetic acid with isopentyl alcohol is 45%, how many grams of isopentyl acetate are formed from 3. H 2SO 4 CAUTION: highly corrosive. We're gonna say 56.

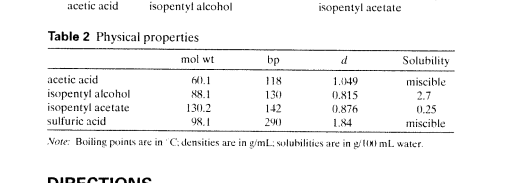
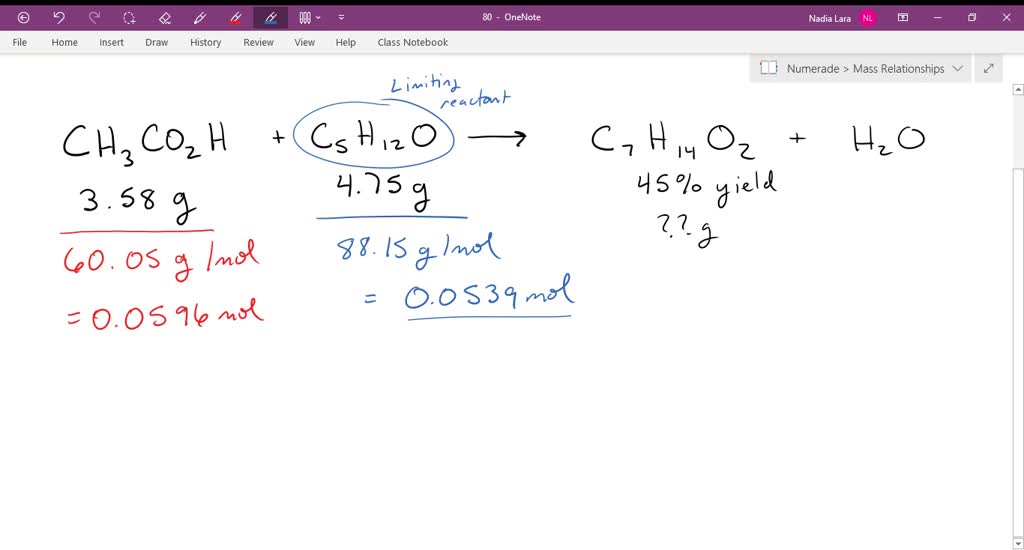
Nam lacinia pulvinar tortor nec facilisis. In our second step we're going to find the theoretical yield, find theoretical yield. I hope this helped, and until next time. Acetic acid CH3CO2H reacts with isopentyl alcohol C5H12O to yield isopentyl acetate C7H14O2 , a fragrant substance with the odor of bananas. In stoichiometry, we can calculate the theoretical yield based on the initial amount of reagents that are present. We used water and sodium bicarbonate to purify the organic layer and then used liquid-liquid extractions to collect it. We're going to convert them all.