Pepsin is a protease enzyme that is produced by the body to aid in the digestion of proteins. It is produced in an inactive form called pepsinogen, which is secreted by the chief cells of the stomach. When pepsinogen is activated, it converts into the active enzyme pepsin, which then goes to work breaking down proteins into smaller peptides.
Pepsinogen is a zymogen, which is a type of enzyme that is produced in an inactive form and requires activation before it can function. Zymogens are found in many different tissues and organs in the body and play important roles in various physiological processes.
Pepsinogen is activated when it comes into contact with hydrochloric acid, which is produced by the parietal cells of the stomach. When the pH of the stomach drops to a certain level, pepsinogen is converted into pepsin. This process is known as autocatalysis, as the pepsinogen itself is responsible for its own activation.
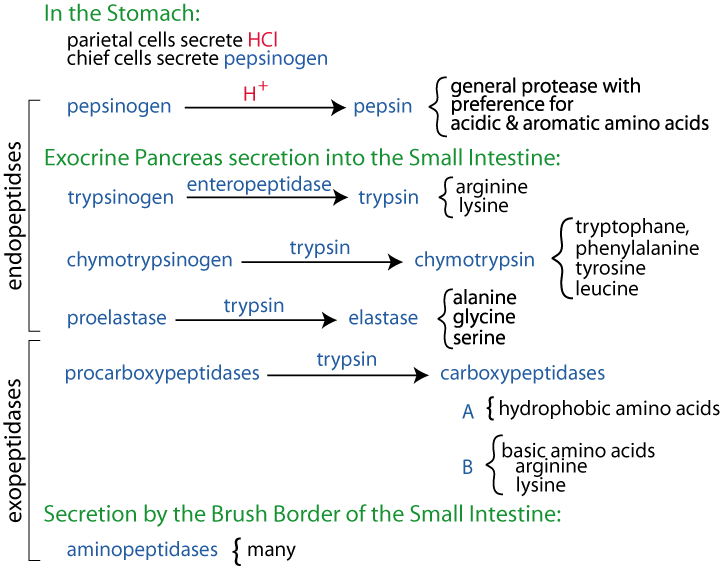
Pepsin is an important enzyme for the digestion of proteins, but it is not the only one. Other proteases such as trypsin, chymotrypsin, and elastase also play a role in breaking down proteins into smaller peptides that can be absorbed by the body.
In addition to its role in digestion, pepsin has also been shown to have other functions in the body. It has been suggested that pepsin may play a role in the immune system, as it can break down proteins on the surface of pathogens and help to destroy them. It has also been proposed that pepsin may have a role in the development of certain types of cancer, although more research is needed to confirm this.
Overall, pepsin is an important enzyme that plays a crucial role in the digestion of proteins in the body. Its inactive form, pepsinogen, is produced by the stomach and activated in the presence of hydrochloric acid. While pepsin is primarily known for its role in digestion, it may also have other functions in the body.
Why is pepsin inactive?

What Happens If Pepsinogen Is Stored In The Small Intestine Too Long? It is the main component of the digestive enzyme pepsin. Specific cells within the gastric lining, known as chief cells, release pepsin in an inactive form, or zymogen form, called pepsinogen. If you have been taking Pepcid brand name for omeprazole to treat heartburn, you should continue to take it unless your doctor tells you otherwise. It is formed in the chief cells of the stomach lining and is one of the most important digestive enzymes in humans and many other animals' digestive systems, where it aids in the digestion of proteins. What is pepsin enzyme? The latest research shows that the functions of Pepsin are far more elaborate than was previously thought. These acids are essential components of DNA and RNA. Working of Pepsin Enzyme Pepsin is expressed as a zymogen called pepsinogen, which has an additional 44 amino acids in its primary structure than the active enzyme.
The reason for producing pepsin in the form of inactive pepsinogen is to
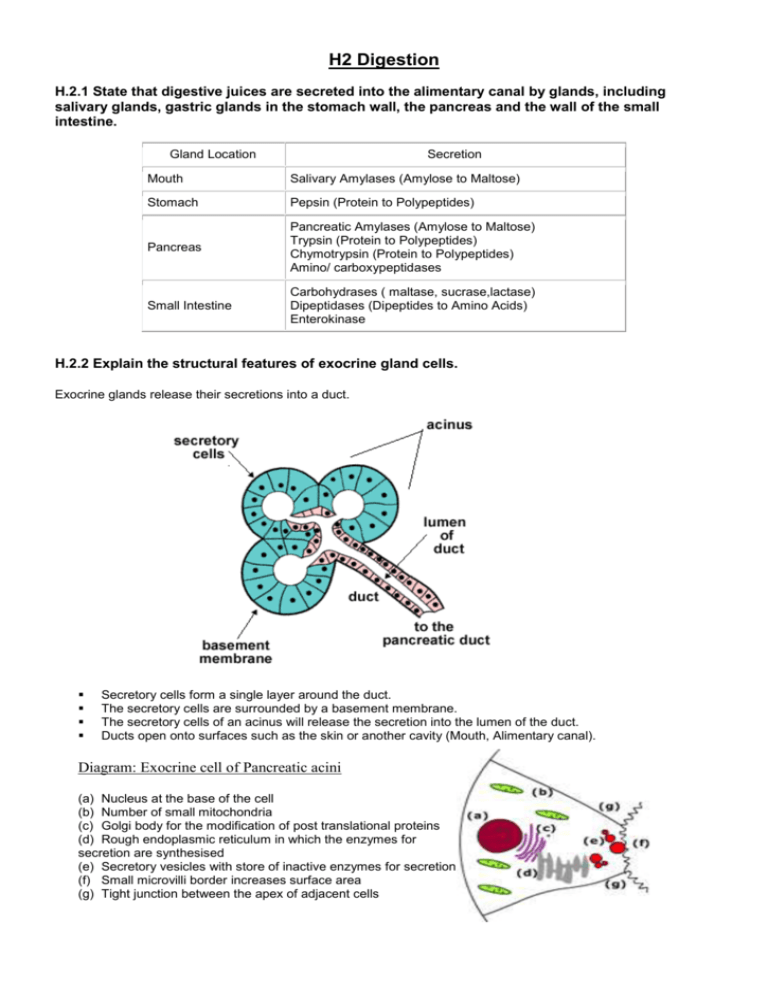
Why is pepsin secreted in an inactive form and then activated after release? Pepsinogen is the inactive form of the enzyme, which is secreted by the parietal or the chief cells of the lining of the stomach. Pancreatitis can cause severe abdominal pain, vomiting, and diarrhea. Pepsinogen is the inactive form of pepsin. The pancreas is close to the small intestine, which is why it is not surprising that the pancreas is affected by gastric bypass surgery. There are different methods to prepare pepsin which is mentioned as Pepsin is made by combining hydrochloric acid with minced stomach linings. Pepsin preferentially hydrolyzes peptide bonds containing the aromatic amino acids' amine group tryptophan, phenylalanine, and tyrosine.
What is the name of the inactive form of pepsin and how is it activated?
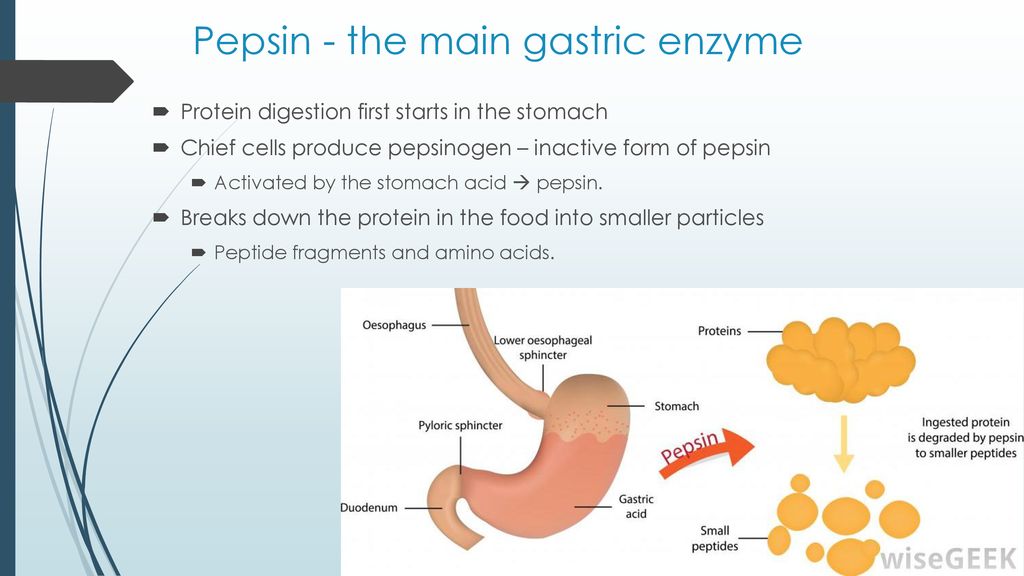
If you stop taking Pepcid without first discussing it with your doctor, you may have a hard time getting off the medication. Why are gastric enzymes inactive until they reach the stomach? Pepsin is a stomach enzyme that aids in the digestion of proteins in food. The main site of protein digestion is the intestine, wherein the trypsin, chymotrypsin secreted by the pancreas , and others work on the digestion of proteins, thereby breaking them down into peptides, which in turn are converted into amino acids. What causes pepsin to change into an active form? Pepsin is inactivated in the duodenum. What role does pepsin play in digestion? What Happens If Pepsinogen Is Released Too Soon? Second, pepsinogen can be stored in the stomach, and it is activated when pepsin is secreted in the intestine. Activation: The inactive form of pepsin, pepsinogen, is activated by HCl of the gastric juice, whilst the inactive form of trypsin, trypsinogen, is activated by an enzyme called enterokinase.