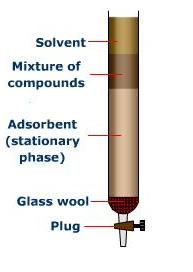
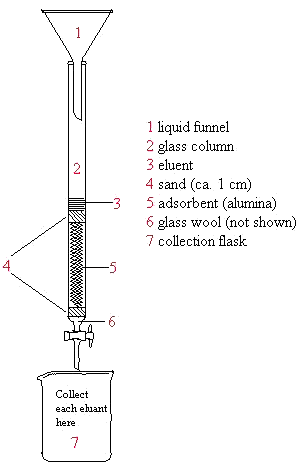
Column chromatography is a common laboratory technique used to separate and purify a mixture of compounds. It is based on the principle of adsorption, in which different compounds in the mixture are attracted to and bind to the surface of a solid stationary phase, while a liquid mobile phase flows through the column.
In a column chromatography experiment, a sample mixture is first prepared and then applied to the top of the column. The mobile phase is then passed through the column, causing the compounds in the mixture to move at different rates as they interact with the stationary phase. As the compounds move through the column, they are separated based on their relative affinity for the stationary phase.
After the mobile phase has passed through the column, the compounds are collected in separate fractions. The fractions can then be analyzed to determine the purity and identity of the compounds present in the mixture.
One of the main advantages of column chromatography is its high separation efficiency. It can be used to separate a wide range of compounds, including proteins, amino acids, and small organic molecules. It is also a relatively simple and inexpensive technique, making it widely used in research and industrial settings.
In conclusion, column chromatography is an important and widely used technique for the separation and purification of mixtures of compounds. It is highly efficient and can be used to separate a wide range of compounds, making it a valuable tool in the laboratory.
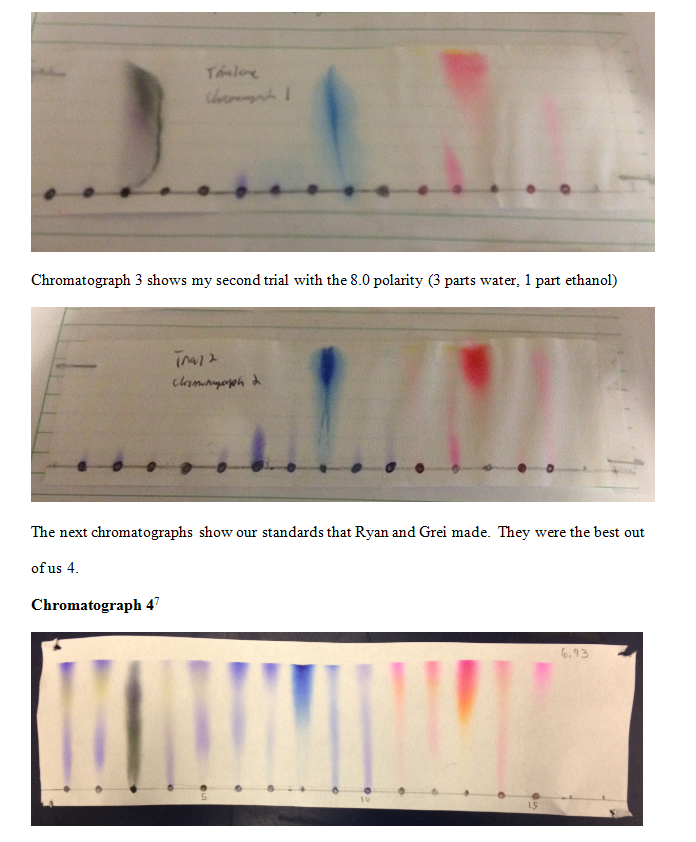
Conclusion In the thin layer chromatography experiment as the mobile phase rose
Also, no reaction with acids or bases or any other solvents was used during the experiment. With the reactions noted, it could be realized that the concentration of the elements depend on the combination that each element has towards each other. In alkaline condition, lignin molecules are converted into negative charge. The pigment that traveled the farthest up on the TLC plate more attracted to the mobile phase, the hexane and ethyl acetate solvent. Lastly, chlorophyll is a green pigment so the green band should have been of chlorophyll while the yellow band should have been of beta-carotene. The green colored food dye is the mixture whose components are separated. Before proceeding with the column chromatography itself, a proper solvent system must be chosen among the different solvents.
Column chromatography conclusion Free Essays

The singlets in RM-09-1 were found at 7. Use of a higher amount of catalyst did not improve the yield while a decrease in the amount of catalyst decreases the yield… XRD Analysis Of Cumnox The SEM result was also in good agreement of XRD analysis. The watchglass test was conducted every two minutes. The movement is based on the polarity of molecules in the sample. In order to identify the compound it was important to separate by dissolving the mixture in an organic solvent which was not soluble in water, and then extracting the solution first with HCl, and then dilute sodium hydroxide solution. What is the main advantage of column chromatography? Column Chromatography What is Column Chromatography? Furthermore, the graph of chlorophyll on the protocol also had more peaks, like our experimental chlorophyll graph Graph 5. From the results, out of the top four pigments that traveled the farthest, the light pink pigment whose Rf was 0.