Hydrogen peroxide is a chemical compound with the formula H2O2. It is a pale blue liquid at room temperature and is commonly used as a disinfectant and bleaching agent. One of the key properties of hydrogen peroxide is its pH level, which can have a significant impact on its effectiveness and safety.
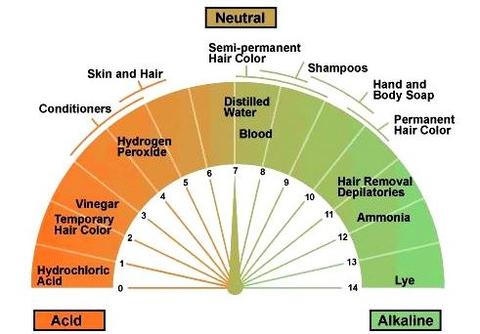
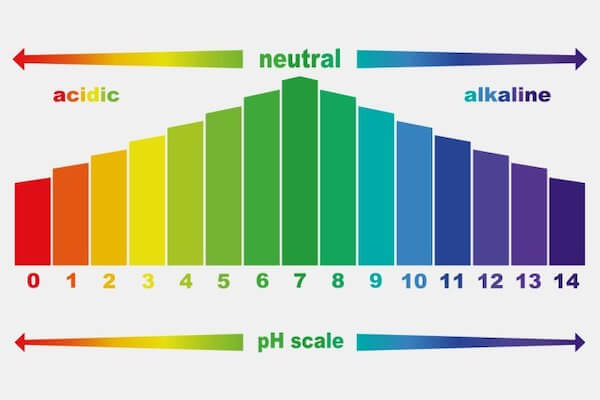
The pH scale is a measure of the acidity or basicity of a solution. It ranges from 0 to 14, with 7 being neutral. A solution with a pH less than 7 is considered acidic, while a solution with a pH greater than 7 is considered basic. The pH of hydrogen peroxide is around 6.5, which makes it slightly acidic.
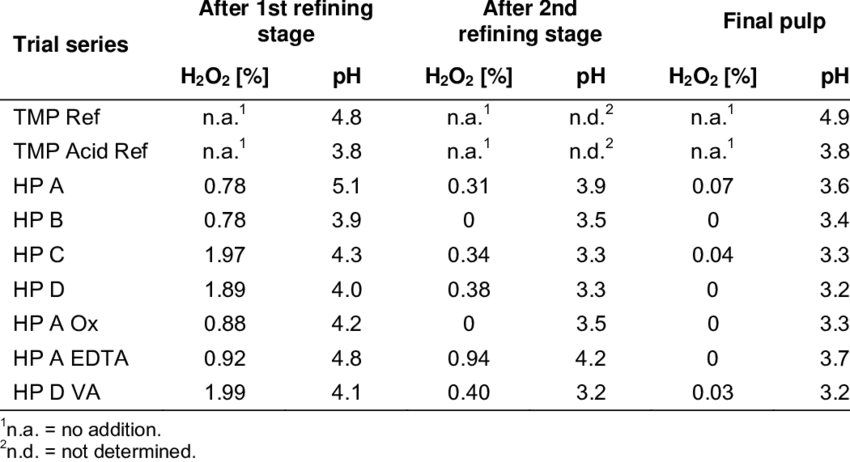
The pH of hydrogen peroxide can vary depending on the concentration of the solution. Lower concentrations of hydrogen peroxide have a higher pH, while higher concentrations have a lower pH. This is because the concentration of hydrogen ions in the solution increases as the concentration of hydrogen peroxide increases.
The pH of hydrogen peroxide can also be affected by the presence of other chemicals in the solution. For example, if hydrogen peroxide is mixed with an acidic substance, the pH of the resulting solution will be lower. On the other hand, if hydrogen peroxide is mixed with a basic substance, the pH of the resulting solution will be higher.
The pH of hydrogen peroxide is important because it can affect its effectiveness as a disinfectant. At a pH of around 6.5, hydrogen peroxide is most effective at killing bacteria and other microorganisms. However, if the pH of the solution is too low or too high, the disinfectant properties of hydrogen peroxide may be reduced.
In addition to its disinfectant properties, the pH of hydrogen peroxide can also affect its safety. If the pH of the solution is too low, it can be corrosive to skin and other tissues. On the other hand, if the pH of the solution is too high, it may not be as effective at killing bacteria and other microorganisms.
In summary, the pH of hydrogen peroxide plays a significant role in its effectiveness and safety. It is important to carefully control the pH of the solution to ensure that it is effective as a disinfectant and safe for use.