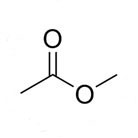
Methyl acetate, also known as MeOAc or acetic acid methyl ester, is a chemical compound commonly used as a solvent. It is a clear, colorless liquid with a fruity odor and is often used in the production of paints, adhesives, and flavors. In this essay, we will discuss the process for preparing methyl acetate through the esterification of acetic acid and methanol.
Before beginning the synthesis of methyl acetate, it is important to gather all necessary materials and equipment. This will include acetic acid, methanol, sulfuric acid, a heating mantle or hot plate, a distillation setup, a thermometer, and a stirring mechanism. It is also important to work in a well-ventilated area and to wear appropriate protective gear, such as goggles and gloves.
The synthesis of methyl acetate begins with the esterification of acetic acid and methanol. To a round-bottomed flask, add a mixture of acetic acid and methanol in a molar ratio of 1:1. For example, if you were using 100 grams of acetic acid, you would also use 100 grams of methanol. Next, add a small amount of sulfuric acid to the flask as a catalyst. The sulfuric acid helps to accelerate the reaction between the acetic acid and methanol.
Next, heat the mixture using a heating mantle or hot plate. The reaction should occur at a temperature between 70-80°C. Stir the mixture constantly to ensure that it is well-mixed and to prevent the formation of hot spots, which could lead to decomposition of the reactants.
Once the reaction is complete, the resulting mixture will contain a combination of methyl acetate and excess methanol. To purify the methyl acetate, the mixture can be distilled. Set up a distillation apparatus, making sure to use a thermometer to monitor the temperature of the distillate. Methyl acetate has a boiling point of 57°C, so collect the distillate until the temperature reaches this point. The resulting liquid should be pure methyl acetate.
In conclusion, preparing methyl acetate involves the esterification of acetic acid and methanol, catalyzed by sulfuric acid. The reaction is heated to a temperature between 70-80°C and stirred to ensure that it proceeds smoothly. The resulting mixture is then distilled to purify the methyl acetate. It is important to follow proper safety precautions when handling chemicals and to work in a well-ventilated area.
How to prepare the following? Methyl acetate (two different ways)

The preparation method is characterized in that the catalyst in the step 1 is an amine type ionic liquid catalyst which is selected from one or more of n-butylamine nitrate, n-butylamine acetate, ethylamine nitrate, ethylamine acetate, propylamine nitrate and propylamine acetate. In the there-necked flask of 500ml, add ethanol 32. When higher catalyst concentrations are employed, shorter reaction times are required for a given conversion. For convenience, the invention will be described herein with reference to a reaction-distillation column. Whenever the reaction vessel is independent from the first distillation column it is preferred to recycle the residue from the base of this column back to the reaction vessel. As stated previously, the methyl acetate production rate is determined by the reactant feed rates.
Methyl Acetate Plant, Methyl Acetate Production Process and Technology Provider

Figure 2 is a schematic diagram of a reaction zone, specifically a reaction-distillation column, useful in this invention and showing its functional sections, i. Bubbling area 50, on both sides of baffle 47, contains a plurality of bubble caps 55. Secondary ester water separator lower floor is methyl chloroacetate layer, removes next rectification cell, obtains qualified product. In product, without Mono Chloro Acetic Acid, therefore, without adopting alkali neutralizing treatment, decomposition and the chloroacetic loss of methyl chloroacetate are also just avoided. When excess methanol is used in the process, the control on the second loop can be eased. Due to the importance of methyl chloroacetate and considerable economic benefit thereof, some medium-sized and small enterprises are produced methyl chloroacetate one after another in recent years. Each tray contains on the order of 150 to 200 bubble caps.
Methyl Acetate Formula

First Mono Chloro Acetic Acid and methyl alcohol are constantly added to continuous esterification in esterifying kettle, water, methyl chloroacetate and the excessive methyl alcohol of generation are constantly distilled out, and part Mono Chloro Acetic Acid is also carried secretly out simultaneously. Common preparation method is direct esterification under sulphuric acid catalysis by Mono Chloro Acetic Acid and methyl alcohol, but it is strong to use concentrated sulfuric acid catalyst to have corrodibility, the problems such as long reaction time, and under normal pressure, reaction yield is 60%. The preferred primary reflux ratios used to provide the control on acetic acid loss overhead whilst effecting the necessary separation may suitably be in the range 1:2 to 10:1, preferably 1:1 to 5:1. Eastman Chemical Co Original Assignee Eastman Kodak Co Priority date The priority date is an assumption and is not a legal conclusion. The tank is preferably located between the reaction section and the methanol stripping section of the reaction-distillation column.
Methyl acetate

Preferably the reaction vessel forms the kettle at the base of the first distillation column. Besides, the esterification heat is made use of as the heating source for vaporization to save energy consumption. Lower floor enters secondary ester water separator 6, opens the 6 water compensating valve moisturizings of secondary ester water separator and carries out secondary extraction, with the each component concentration of gas chromatographic detection levels. Heat can be supplied to the reaction- distillation column by conventional means. If necessary, fractional distillation is used to further purify it. This instrument should have a high water concentrate alarm. A process according to claim 1 wherein methanol, acetic acid and entrainer are continuously fed to an esterification reaction vessel containing entrainer, acetic acid and esterification catalyst at elevated temperature thereby producing a product comprising entrainer, methyl acetate and water, distilling from the product in a first distillation column an overhead fraction comprising methyl acetate, water and entrainer, condensing the overhead fraction, separating at least part of the overhead fraction by decantation into a lower aqueous phase and an upper organic phase containing methyl acetate and entrainer and thereafter separating the organic phase by distillation in a second distillation column into an overhead methyl acetate fraction and a residue entrainer fraction.
EP0060717A1

Patent 1,400,849 which produces highly pure methyl acetate with high reactant conversions. The present invention is mainly for catalyst residue in product, destroy product stability, thus yield is declined, therefore have developed a kind of novel high-activity, amine type ion urges liquid agent, catalyst type is as follows: the amine type ionic liquids such as nitric acid n-Butyl Amine 99, acetic acid n-Butyl Amine 99, nitric acid ethamine, acetate triethylamine, nitric acid propylamine, acetic acid propylamine. Under normal operation, this catalyst has an average service life of three years or longer. The acetic acid of 50% massfraction is added till solution is slightly acidic in filtrate. It should be kept in a cool environment. In this way, the concentration of the product in the reactor is increased, thereby the reaction rate is boosted, moreover the incurrence of side reactions is inhibited due to the isolation of product out of the reactor in time.