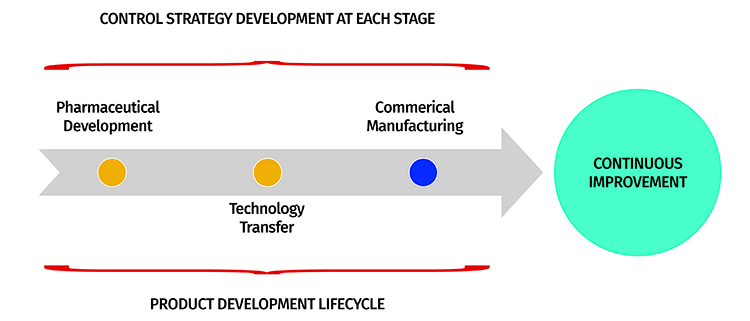
The drug product life cycle refers to the stages of development and commercialization of a pharmaceutical product. It begins with the discovery and development of a new drug and ends with the withdrawal or discontinuation of the product from the market. Understanding the drug product life cycle is important for pharmaceutical companies, regulatory agencies, and healthcare professionals as it helps to ensure the safety, efficacy, and quality of the product throughout its lifespan.
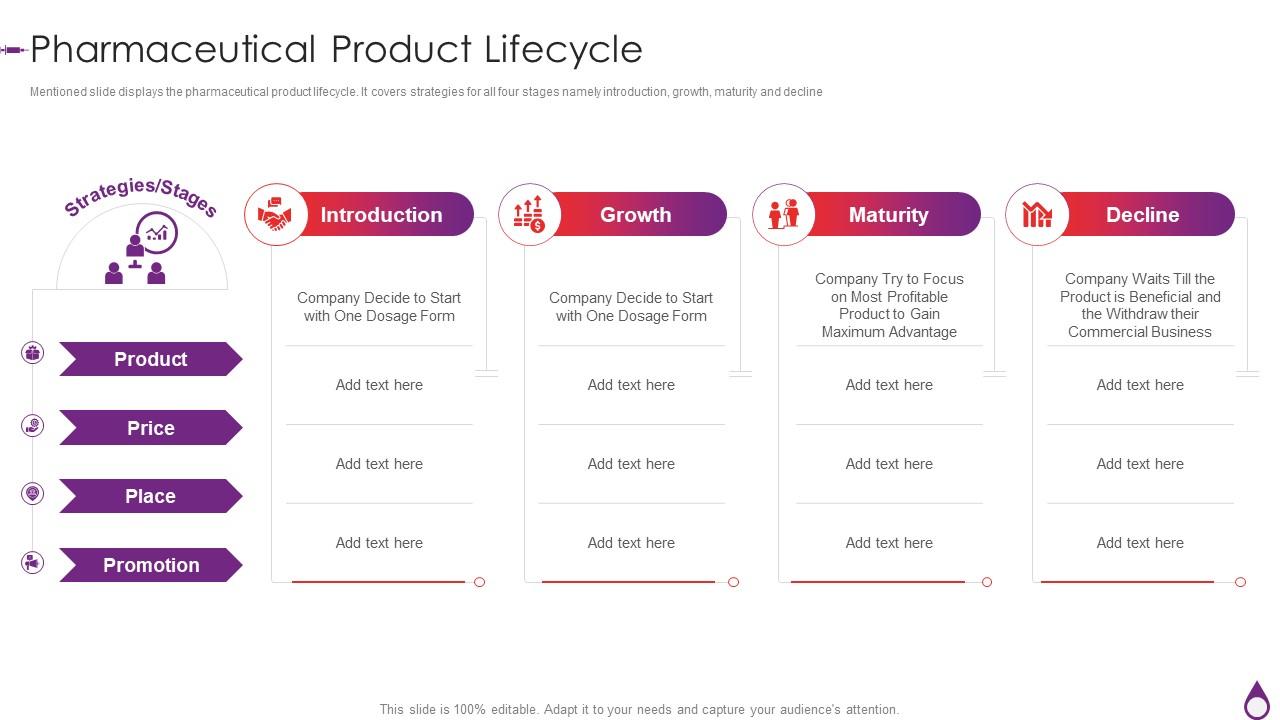
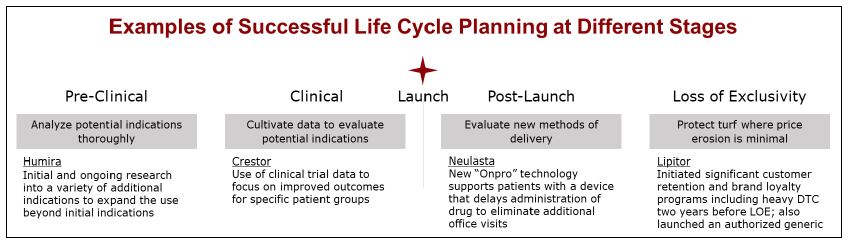
The first stage of the drug product life cycle is the discovery and development phase. This is the research and development phase where scientists and researchers identify new compounds or substances that have the potential to be developed into a drug. This process can involve screening thousands of compounds to identify those with the desired therapeutic effects and suitable pharmacokinetic properties. Once a compound has been identified, it undergoes preclinical testing to determine its safety and effectiveness in animals. If the compound shows promise in these tests, it can then move on to clinical trials in humans.
The second stage of the drug product life cycle is the clinical trial phase. Clinical trials are research studies that involve human volunteers and are conducted to determine the safety and effectiveness of a new drug. Clinical trials are conducted in three phases: Phase 1, Phase 2, and Phase 3. Phase 1 clinical trials are the first studies in humans and typically involve a small number of healthy volunteers. The primary goal of Phase 1 clinical trials is to determine the safety and tolerability of the drug. Phase 2 clinical trials involve a larger number of patients with the condition being treated and are designed to evaluate the effectiveness of the drug and to identify any potential side effects. Phase 3 clinical trials involve an even larger number of patients and are designed to confirm the effectiveness of the drug, monitor side effects, and compare the drug to other treatments.
The third stage of the drug product life cycle is the regulatory review and approval phase. If the clinical trials are successful, the drug manufacturer submits a New Drug Application (NDA) to the regulatory agency, such as the US Food and Drug Administration (FDA), for review and approval. The regulatory agency reviews the data from the clinical trials, as well as the manufacturing process and the proposed labeling for the drug, to determine whether the drug is safe and effective for its intended use. If the drug is approved, it can be marketed and sold to the public.
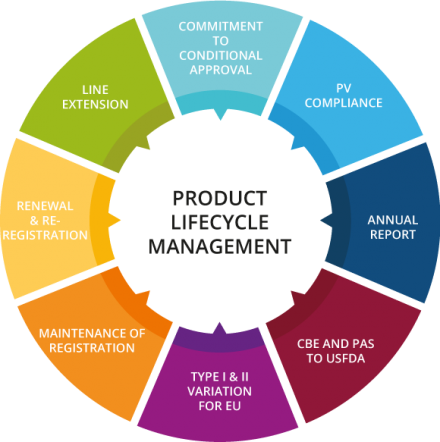
The fourth stage of the drug product life cycle is the post-marketing phase. Once a drug has been approved and is on the market, the manufacturer is required to monitor the safety of the drug and report any adverse events to the regulatory agency. The manufacturer may also conduct additional studies to gather more information about the long-term safety and effectiveness of the drug.
The final stage of the drug product life cycle is the withdrawal or discontinuation of the drug from the market. This can occur for a variety of reasons, such as the expiration of the drug's patent, the introduction of a newer and more effective treatment, or the identification of serious safety concerns. When a drug is withdrawn or discontinued, it is no longer available for prescription or purchase.
In conclusion, the drug product life cycle is a complex process that involves many stages and parties. It is essential for ensuring the safety, efficacy, and quality of pharmaceutical products throughout their lifespan. By understanding the drug product life cycle, pharmaceutical companies, regulatory agencies, and healthcare professionals can work together to bring new and innovative treatments to the market and ensure that they are used safely and effectively to improve the health and well-being of patients.