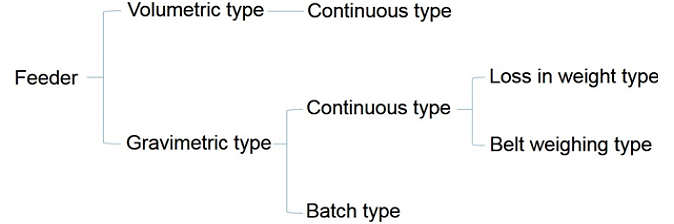
Volumetric and gravimetric analysis are two important methods used in chemical analysis to determine the concentration or amount of a substance present in a sample. Both methods involve measuring the mass or volume of a substance, but they differ in the way they do it and the type of information they provide.
In volumetric analysis, the volume of a solution containing a known concentration of a substance is measured and used to calculate the concentration of the substance in the original sample. This method relies on the fact that the volume of a solution is directly proportional to the number of moles of solute it contains. For example, in titration, a solution of known concentration (titrant) is added to a sample (analyte) until a chemical reaction occurs, indicating that the analyte has been completely reacted. The volume of titrant added is used to calculate the concentration of the analyte. Volumetric analysis is often used in quantitative analysis, where the goal is to determine the exact amount of a substance present in a sample.
On the other hand, gravimetric analysis involves the measurement of mass to determine the concentration or amount of a substance in a sample. This method relies on the fact that the mass of a substance is directly proportional to the number of moles it contains. In gravimetric analysis, the substance is first separated from the sample by a chemical reaction, filtration, or other means. The mass of the purified substance is then measured and used to calculate the concentration or amount of the substance in the original sample. Gravimetric analysis is often used in qualitative analysis, where the goal is to identify the presence or absence of a substance in a sample.
There are several differences between volumetric and gravimetric analysis. One major difference is the type of measurement used. Volumetric analysis uses volume as the measure of concentration, while gravimetric analysis uses mass. Another difference is the type of information provided. Volumetric analysis provides quantitative information about the amount of a substance present in a sample, while gravimetric analysis provides qualitative information about the presence or absence of a substance. Finally, volumetric analysis is generally more accurate and precise than gravimetric analysis, due to the difficulty of accurately measuring and purifying small amounts of substance in gravimetric analysis.
In summary, volumetric and gravimetric analysis are two important methods used in chemical analysis to determine the concentration or amount of a substance in a sample. Volumetric analysis uses volume as the measure of concentration and provides quantitative information about the amount of a substance present in a sample, while gravimetric analysis uses mass and provides qualitative information about the presence or absence of a substance. Both methods have their own advantages and limitations, and the choice of method depends on the specific requirements of the analysis.