Peroxide value is a measure of the concentration of peroxides in a substance, typically an oil or fat. Peroxides are compounds that contain an oxygen-oxygen bond, and they can be harmful to both human health and the stability of the substance in which they are present. Therefore, it is important to determine the peroxide value of oils and fats in order to ensure their quality and safety.
One way to determine the peroxide value of a substance is through titration. Titration is a laboratory technique in which a known concentration of a reagent, called the titrant, is added to a sample until a chemical reaction is complete. The volume of titrant needed to complete the reaction is then used to calculate the concentration of the substance being measured.
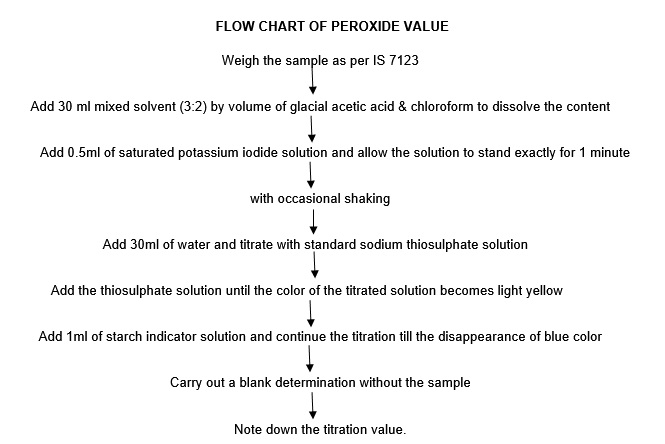
To determine the peroxide value of an oil or fat using titration, a sample of the substance is first placed in a flask or beaker. An indicator solution, such as potassium iodide, is then added to the sample. The indicator solution reacts with the peroxides in the sample to produce iodine, which changes the color of the solution.
Next, a standardized solution of sodium thiosulfate, also known as a titrant, is added to the flask or beaker. The sodium thiosulfate reacts with the iodine to produce a colorless solution. The volume of sodium thiosulfate needed to fully react with the iodine is measured using a burette, a precision instrument used to measure the volume of a liquid.
The peroxide value of the sample is then calculated using the following formula:
Peroxide value (meq/kg) = (Volume of sodium thiosulfate needed / Sample weight) x Normality of sodium thiosulfate
The normality of the sodium thiosulfate is a measure of its concentration, and it is typically provided with the standardized solution. The sample weight is typically measured in kilograms.
It is important to note that the peroxide value determined through titration is a measure of the concentration of peroxides present in the sample at the time of the analysis. Peroxides can continue to form over time, so the peroxide value of an oil or fat can change over its shelf life. Therefore, it is important to regularly test the peroxide value of oils and fats to ensure their quality and safety.
In conclusion, the determination of peroxide value by titration is a useful and precise method for measuring the concentration of peroxides in oils and fats. This information is important for ensuring the quality and safety of these products and can help to protect the health of consumers.

+T+x+1000+meq%2FKg.jpg)




