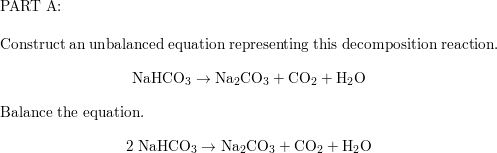Sodium hydrogen carbonate, also known as baking soda, is a commonly used chemical compound with the chemical formula NaHCO3. It is a white, crystalline solid that is commonly used as a food ingredient, a cleaning agent, and a fire suppressant.
One interesting aspect of sodium hydrogen carbonate is its ability to undergo decomposition, which is the process of breaking down a compound into simpler substances. There are several ways in which sodium hydrogen carbonate can decompose, and these decomposition reactions can be either thermal or chemical in nature.
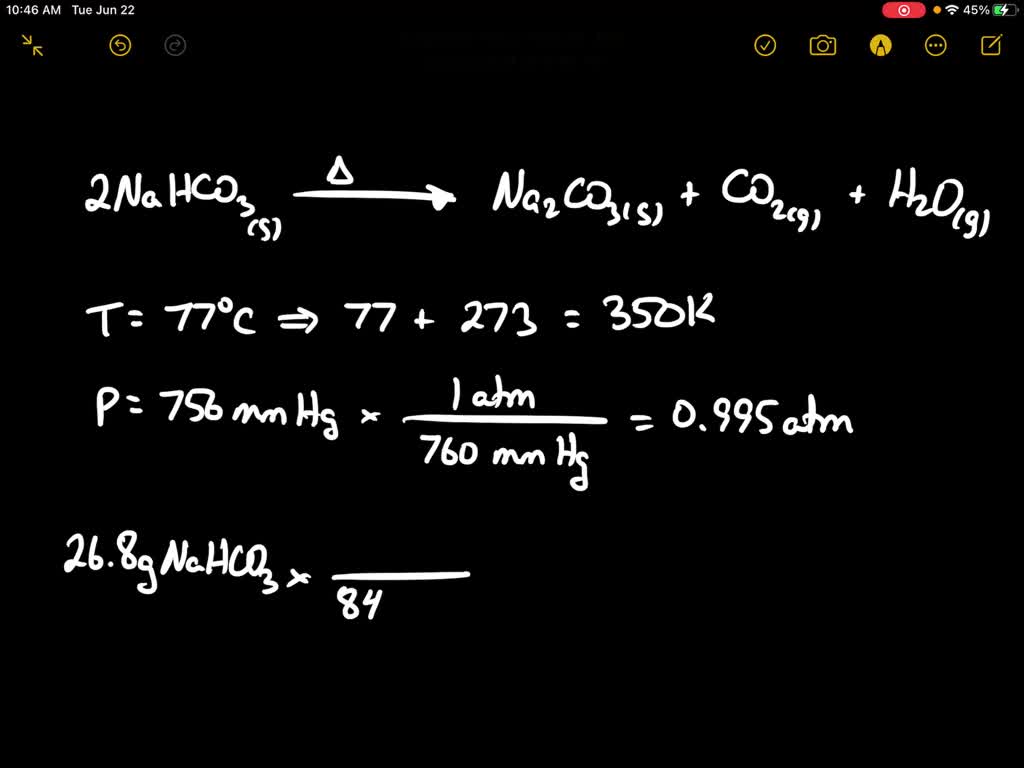
Thermal decomposition of sodium hydrogen carbonate occurs when the compound is heated to high temperatures, typically above 300°C. When this happens, the compound decomposes into sodium carbonate (Na2CO3), water (H2O), and carbon dioxide (CO2). The chemical equation for this reaction is:
2 NaHCO3 -> Na2CO3 + H2O + CO2
Chemical decomposition of sodium hydrogen carbonate can also occur in the presence of certain chemicals, such as acids. When sodium hydrogen carbonate is mixed with an acid, it reacts to form salt, water, and carbon dioxide. The chemical equation for this reaction is:
NaHCO3 + HCl -> NaCl + H2O + CO2
Decomposition of sodium hydrogen carbonate is an important chemical reaction with numerous practical applications. For example, the thermal decomposition of sodium hydrogen carbonate is used in the production of sodium carbonate, which is a widely used industrial chemical. The chemical decomposition of sodium hydrogen carbonate is also used in the production of baking powder, which is a mixture of sodium hydrogen carbonate and an acid.
In conclusion, decomposition is an important property of sodium hydrogen carbonate, and it can occur through both thermal and chemical means. This decomposition reaction has numerous practical applications, including the production of sodium carbonate and baking powder.