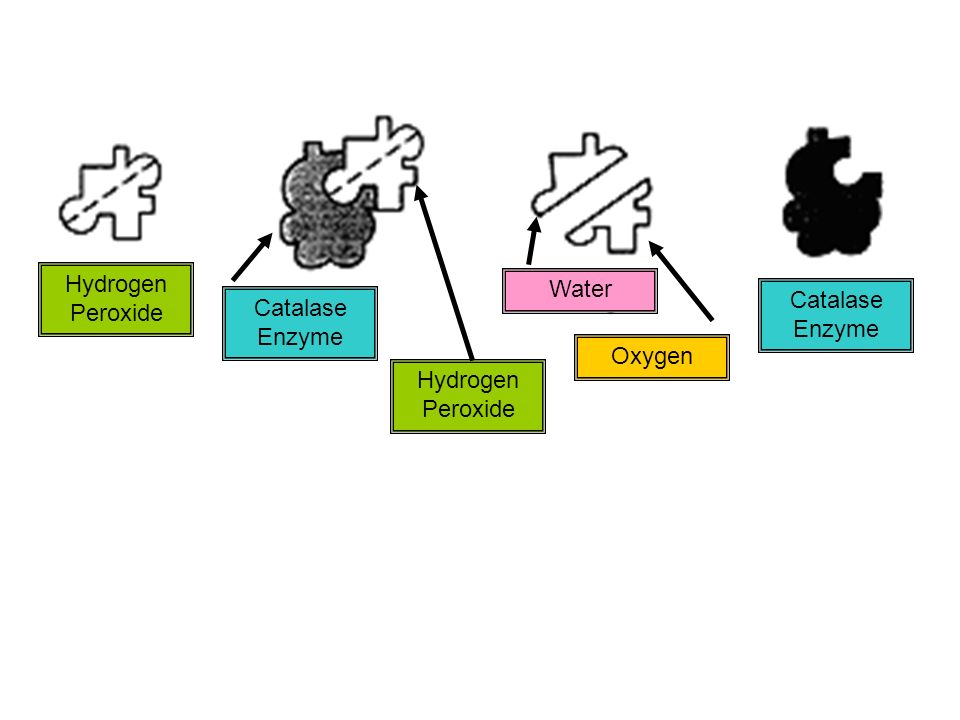
Catalase is an enzyme that is found in most living organisms and is responsible for catalyzing the breakdown of hydrogen peroxide (H2O2) into water and oxygen. This reaction is important because hydrogen peroxide is a toxic byproduct of cellular metabolism and can cause cell damage if it accumulates.
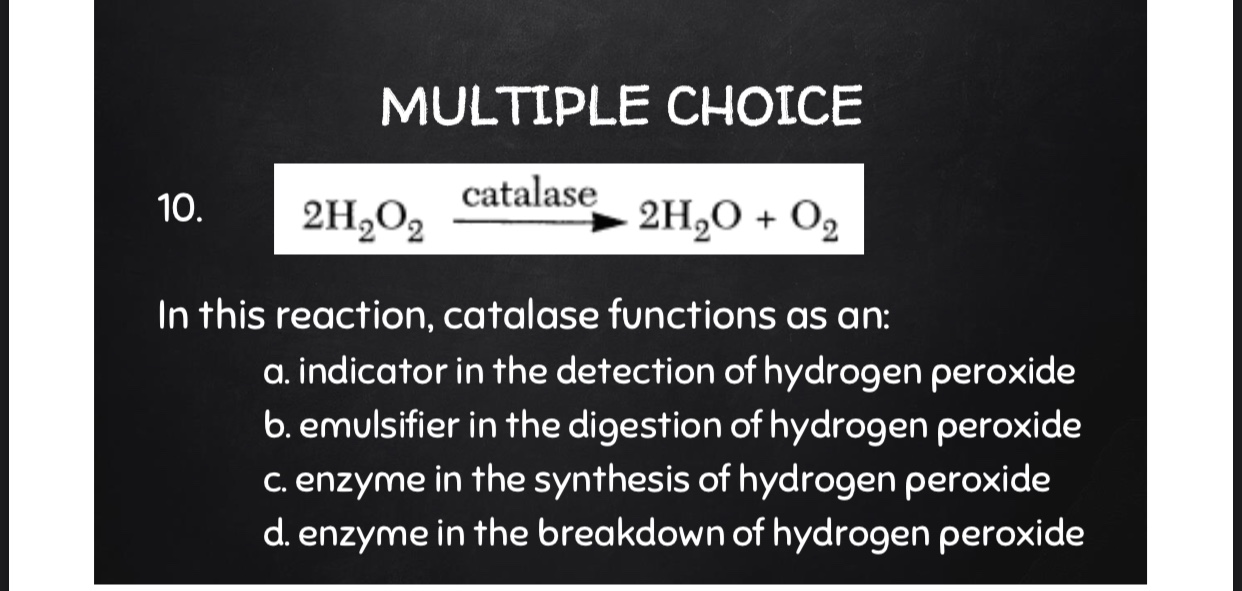
The reaction catalyzed by catalase can be represented by the following equation:
2H2O2 → 2H2O + O2
In this reaction, two molecules of hydrogen peroxide are converted into two molecules of water and one molecule of oxygen. The oxygen is released as a gas, which is why the reaction is often accompanied by the production of bubbles.
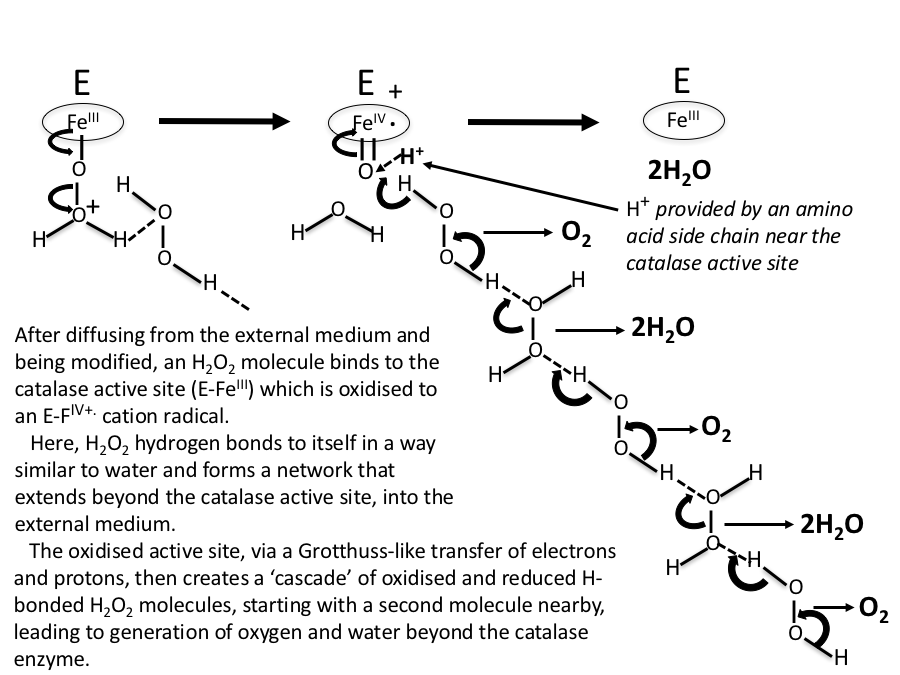
The catalase enzyme is a large protein that is composed of four subunits, each with its own active site where the reaction takes place. The active site contains a heme group, which is a type of molecule that contains iron and is responsible for the enzyme's catalytic activity.
The reaction catalyzed by catalase is extremely fast, with a rate that is millions of times faster than the uncatalyzed reaction. This is due to the presence of the active site, which provides a specific environment for the reactants to interact and promotes the formation of the products.
Catalase is found in high concentrations in organs such as the liver and kidney, where it helps to detoxify the body by breaking down hydrogen peroxide that is produced as a byproduct of cellular metabolism. It is also found in smaller amounts in other tissues, including the brain and muscles, where it plays a role in protecting cells from oxidative stress.
In conclusion, catalase is an important enzyme that helps to protect cells from the toxic effects of hydrogen peroxide by catalyzing its breakdown into water and oxygen. Its high catalytic activity and widespread distribution in the body make it an essential enzyme for maintaining the health and function of cells.
About The Equation For The Catalase Reaction With Hydrogen Peroxide Free Essay Example
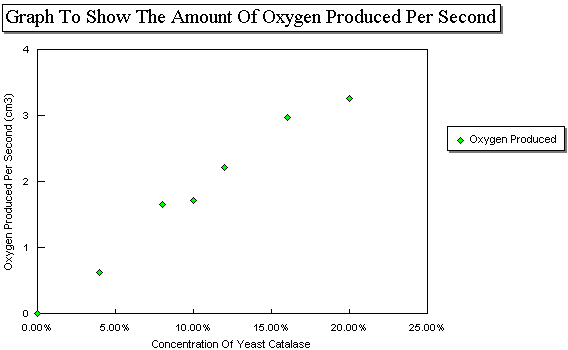
When the concentration of enzyme increases, the rate of reaction increases until a maximum velocity is reached. Get Started It is very important like the substrate that comes and you possess to the energetic site and also the key best suited into the fasten. Using a potato and hydrogen peroxide, we can observe how enzymes like catalase work to perform decomposition, or the breaking down, of other substances. The preparation of blended potato in a beaker which is exposed to the air should be prevented because oxidation will occur and this may affect the activity of enzyme catalase in it. The enzyme is the lock and the key is the substrate; only the correct key could fit into the keyhole of the lock. On the other side, when the temperature or pH is optimum for the reaction, the rate of reaction is the highest. Take care inserting the bung in the conical flask- it needs to be a tight fit, so push and twist the bung in with care.
Reaction of Catalase with Hydrogen Peroxide Composition Essay
The variables are as follows: — Independent variable: this is the only feature that will change throughout the experiment. Then, the slope of increasing line becomes less steep with further increase in concentration of enzyme. However, throughout the experiment some errors might occur in which the real values may not be obtained. Using the 25ml measuring canister, measure 5cm? Push the plunger on the syringe and immediately start the stopclock. To protect itself, the body makes catalase, the enzyme that decomposes hydrogen peroxide before it can form hydroxyl radicals.
Investigating an enzyme
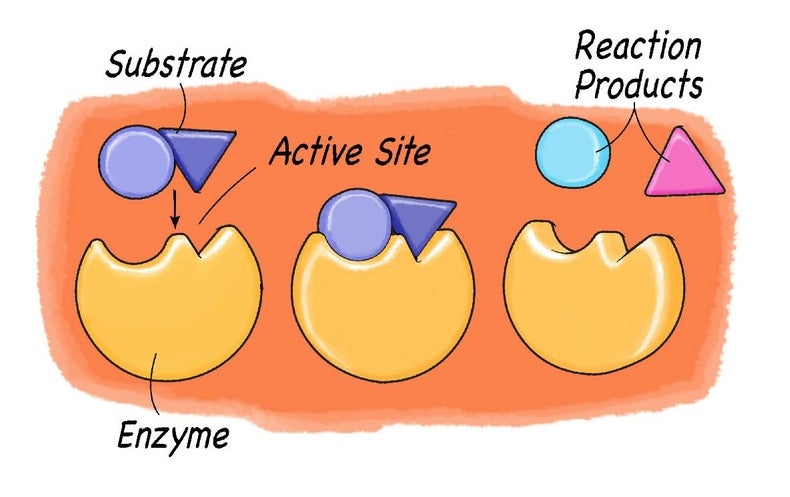
In this case, enzyme catalse has a specific active site, which just for the substrate hydrogen peroxide to fit in, then the reaction takes place. The active site is very specially shaped and only will work on substrates that match. This type of enzyme accelerates reactions in which a substrate is broken down into simpler compounds through reaction with adding up water molecules. Use this information as a check on the practical set-up. The catalase enzyme breaks down hydrogen peroxide, which is a byproduct of some cell functions, into oxygen. After this, oxygen is given off at a steady rate which slowly decreases in the course of an hour. In the channel is a heme group which is a iron molecule bound to the center of a ring-like structure called a porphyrin ring.