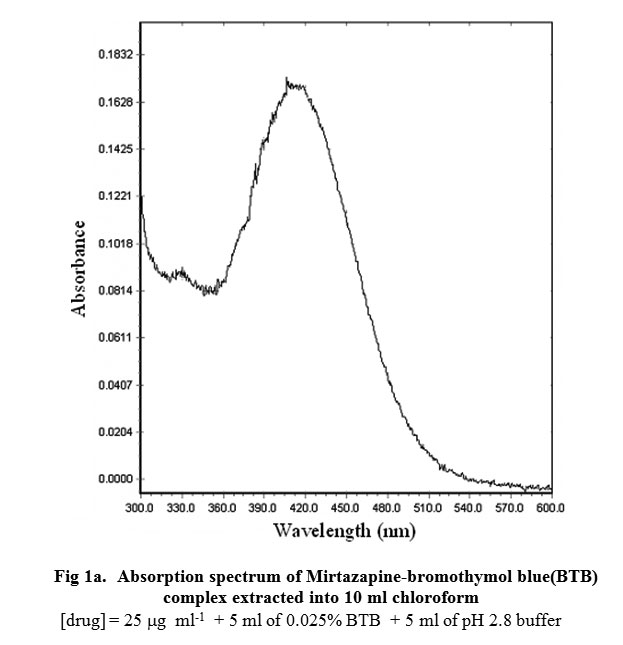
Bromophenol blue is a compound that is commonly used as a pH indicator in spectrophotometry, a technique that measures the amount of light absorbed by a substance. Spectrophotometry is a widely used analytical tool in many fields, including chemistry, biochemistry, and environmental science, among others.
Bromophenol blue is a dye that changes color in response to changes in pH. At a pH of 3.0, it appears yellow, while at a pH of 4.6, it appears blue. This property makes it useful as a pH indicator in spectrophotometry, as the change in color can be easily measured using a spectrophotometer.
To use bromophenol blue in spectrophotometry, a solution of the compound is prepared in a cuvette, which is a small, clear glass or plastic tube used to hold samples for analysis. The cuvette is placed in the spectrophotometer, which measures the amount of light absorbed by the solution at a specific wavelength. The absorbance of the solution is then plotted against the concentration of the substance being measured, which allows for the determination of the concentration of the substance in the sample.
One of the advantages of using bromophenol blue in spectrophotometry is that it is a highly sensitive indicator, meaning that it can detect very small changes in pH. This makes it useful for applications where precise measurements are required, such as in biochemistry, where pH plays a critical role in many biological processes.
In addition to its use as a pH indicator, bromophenol blue is also used as a dye in the production of textiles, plastics, and paper, as well as in the manufacture of cosmetics and other personal care products. It is also used in the laboratory as a tracking dye in electrophoresis, a technique used to separate molecules based on their size and charge.
Overall, bromophenol blue is a valuable compound that has many important applications in a variety of fields, including spectrophotometry, where it is used as a pH indicator. Its sensitivity, ease of use, and versatility make it a valuable tool for researchers and analysts working in a wide range of fields.

+Set+up+8+test+tubes+as+follows%3A.jpg)