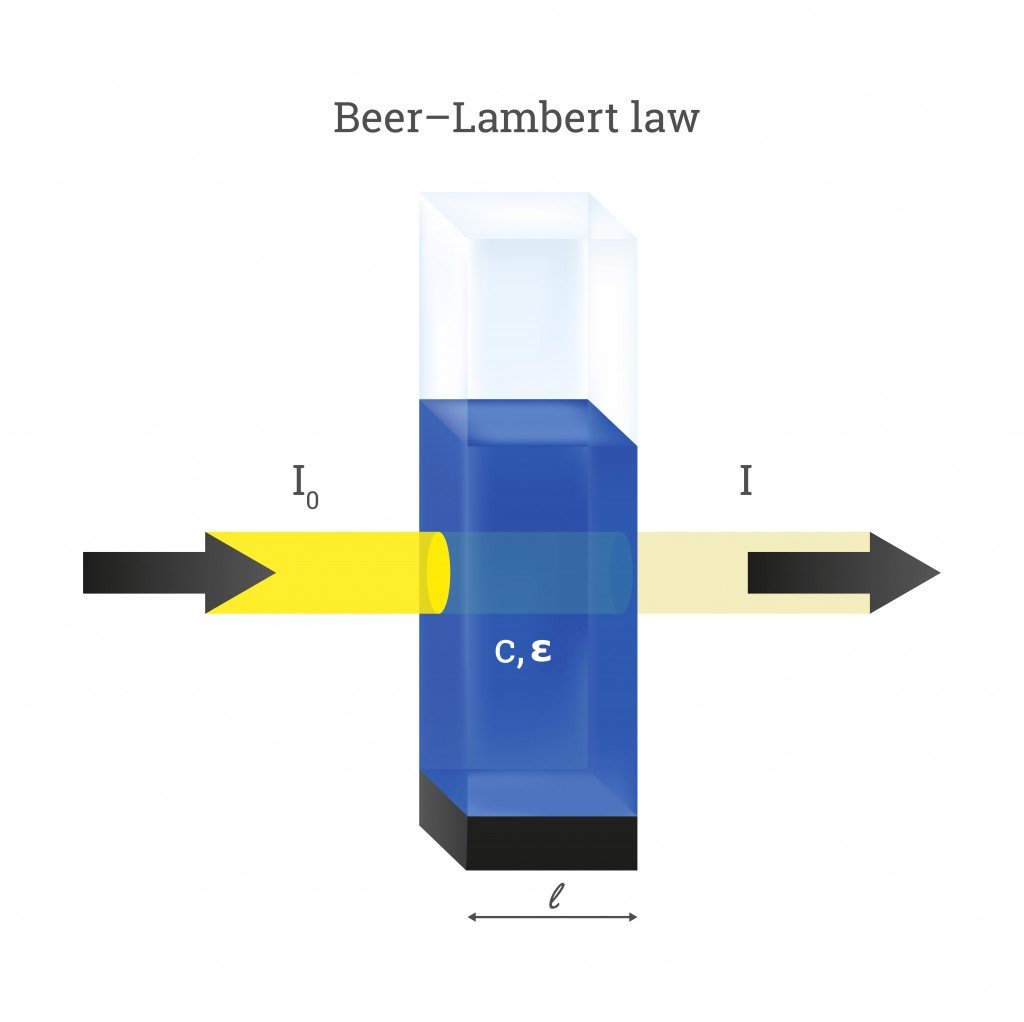
The Beer-Lambert law, also known as the Beer-Lambert-Bouguer law or the Beer's law, is a fundamental principle in spectrophotometry that describes the relationship between the absorption of a substance and the concentration of that substance in a solution. This law states that the absorption of a compound by a solution is directly proportional to the concentration of the compound in the solution and the path length of the light through the solution.
The law is expressed mathematically as:
A = ε * b * c
where A is the absorbance of the solution, ε is the molar extinction coefficient of the compound, b is the path length of the light through the solution, and c is the concentration of the compound in the solution.
The Beer-Lambert law is a crucial tool in the field of analytical chemistry, as it allows researchers to determine the concentration of a compound in a solution by measuring its absorbance. This is useful in a wide range of applications, including the analysis of environmental samples, food and beverage quality control, and the development of pharmaceuticals.
One of the main advantages of the Beer-Lambert law is that it is relatively simple to use and requires only a spectrophotometer, which is a device that measures the absorbance of a substance at a specific wavelength. To use the Beer-Lambert law, researchers simply measure the absorbance of a solution containing the compound of interest at a specific wavelength, and then use the equation above to calculate the concentration of the compound in the solution.
There are some limitations to the Beer-Lambert law, however. One limitation is that it is only applicable to solutions that are dilute enough that the absorbance of the compound is not affected by the presence of other compounds in the solution. In addition, the Beer-Lambert law only holds true for monochromatic light, or light of a single wavelength. This means that it is not applicable to solutions that absorb light at multiple wavelengths, or to solutions that contain multiple absorbing compounds.
Despite these limitations, the Beer-Lambert law is an important principle in spectrophotometry and continues to be widely used in a range of scientific and technological applications. Its simplicity and versatility make it an invaluable tool for researchers seeking to understand the properties and concentrations of compounds in solution.
Beer Lambert Law

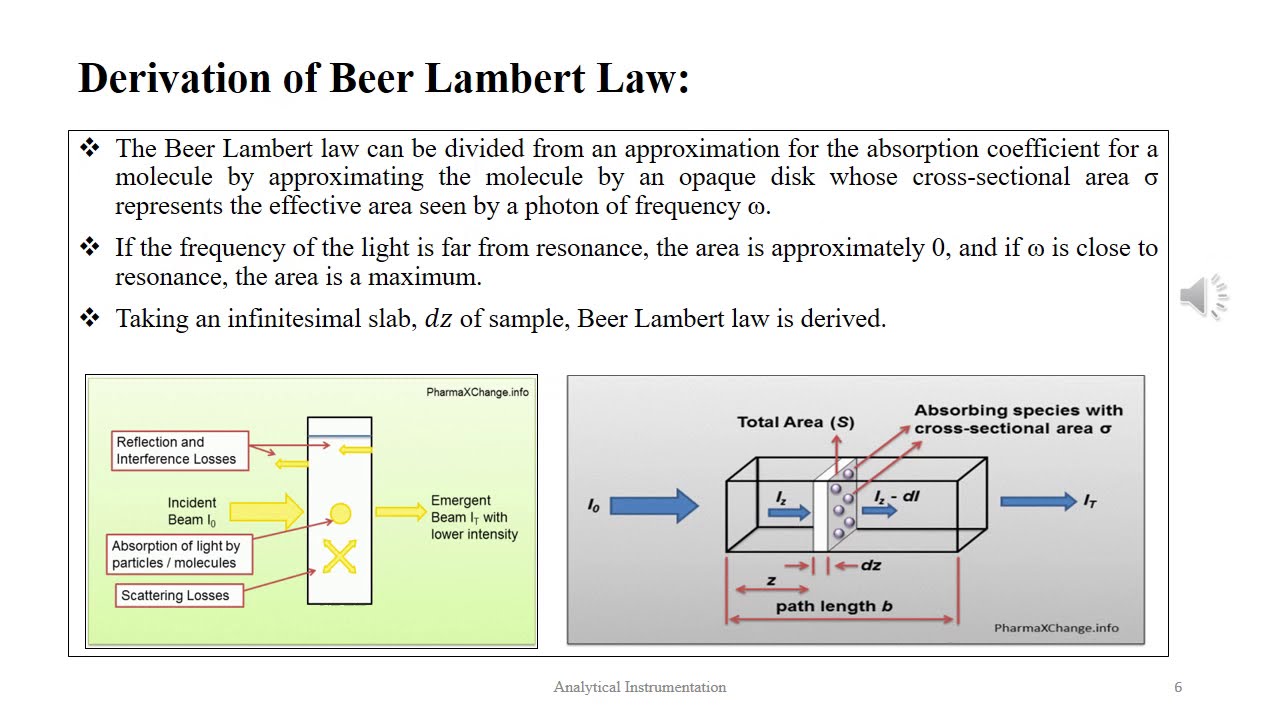
At first sight, one might think that the intensity of light should decrease in arithmetical progression, but Bouguer argues that this cannot be true. Chapman and Hall Ltd. To the analysis menu. Therefore, the wavelength of maximum absorption by a substance is one of the characteristic properties of that material. As noticed by Bouguer, the logarithmic curve, which is well known to mathematicians and surveyors, has the properties that the subtangent is constant and, most importantly in the present case, the ordinates decrease in geometrical progression. In this way, the intensity as a function of depth can be described by the logarithmic curve QRXY, where BC is the axis of abscissae the x-axis.
11.1.1: Beer
Bouguer then goes on, giving several examples of how transmission curves can be constructed and used in various problems. Annalen der Physik und Chemie in German. The mathematical derivation of the Beer-Lambert law is as follows. There are at least six conditions that need to be fulfilled in order for the Beer—Lambert law to be valid. Son of a poor tailor in Mühlhausen, Elsass Alsace , he had to leave school at the age of twelve to help his father with tailoring. } Therefore, measurements at two wavelengths yields two equations in two unknowns and will suffice to determine the amount concentrations c 1 and c 2 as long as the molar attenuation coefficient of the two components, ε 1 and ε 2 are known at both wavelengths. Point to Remember: Here, the absorbance of the material will be measured at the wavelength at which we would observe the maximum absorption, and the temperature will be kept at a uniform level.
Beer
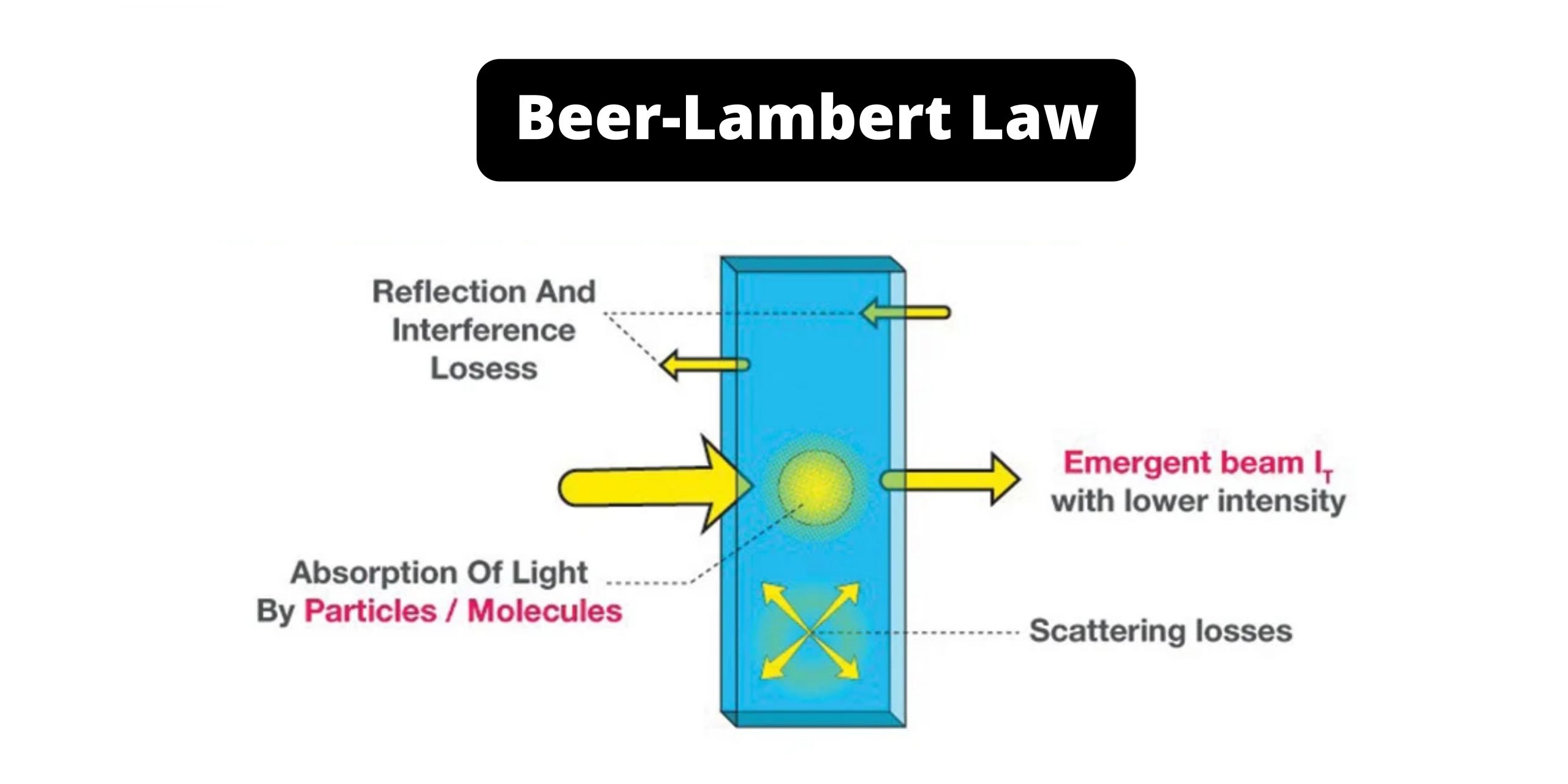
Also, Iz is the strength with which z enters the i nfinitesimal. Different substances absorb different wavelengths of light. This is the reason for the directly proportional relationship between the absorbance and length of the sample. Notice that there are no units given for absorptivity. Absorbance isn't very good for making comparisons The importance of concentration The proportion of the light absorbed will depend on how many molecules it interacts with. However, in the case of a dilute solution, the number of molecules to react with the incident light will be far less, hence, the attenuation of the incident light will be less. For example, ethanal has two absorption peaks in its UV-visible spectrum - both in the ultra-violet.






+is+usually+1+cm+(for+standard+cuvettes)..jpg)