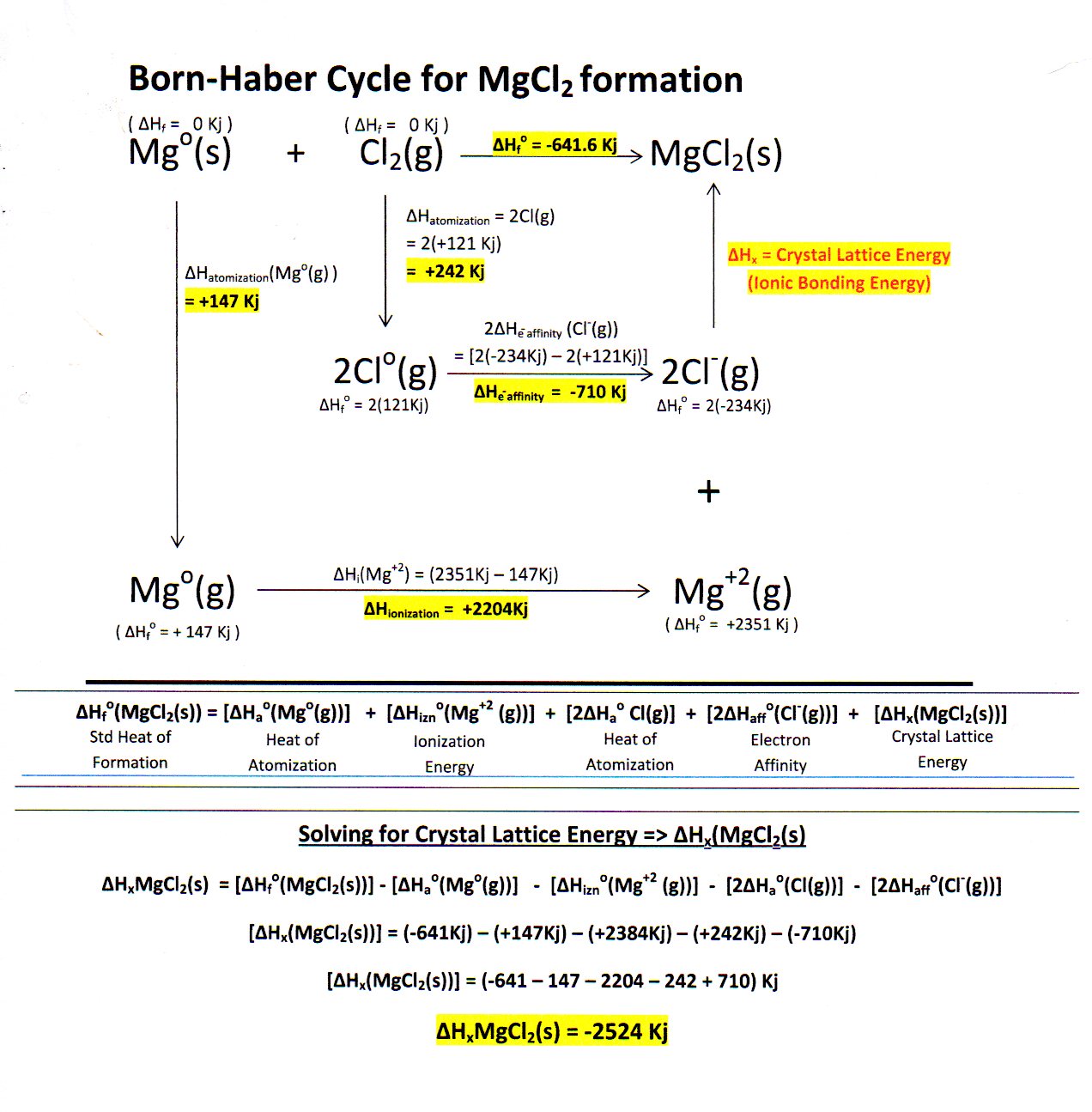
Magnesium oxide is a chemical compound with the formula MgO. It is composed of magnesium and oxygen atoms, and is a white, powdery solid.
Magnesium is a metal found in the earth's crust, and is the eighth most abundant element in the world. It is a silvery-white metal that is highly reactive, meaning that it reacts easily with other elements. Magnesium is often found in compounds with oxygen, such as in the form of magnesium oxide.
Oxygen is a chemical element with the symbol O and atomic number 8. It is a highly reactive nonmetal, and is the third most abundant element in the universe. Oxygen is a gas at room temperature and is essential for life on Earth.
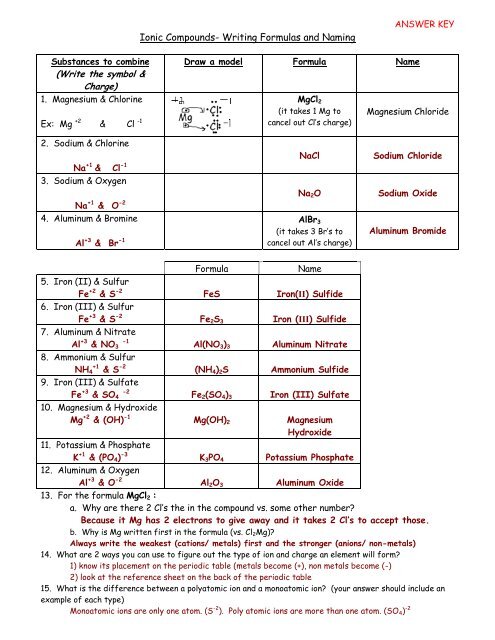
The chemical formula for magnesium oxide is MgO, which indicates that the compound contains one magnesium atom and one oxygen atom. The compound is typically written with the magnesium atom first, followed by the oxygen atom, and the subscripts indicate the number of atoms of each element present in the compound. In this case, the subscripts are not needed, as there is only one atom of each element present.
Magnesium oxide has a number of important uses in industry and medicine. It is used as a refractory material, meaning that it can withstand high temperatures and is used in the construction of furnaces and kilns. It is also used as an antacid, as it neutralizes stomach acid, and is used to treat heartburn and indigestion. Magnesium oxide is also used in the production of ceramics, cement, and other building materials.
In conclusion, the formula for the compound magnesium oxide is MgO. This compound is composed of one magnesium atom and one oxygen atom, and has a number of important uses in industry and medicine.
Magnesium Oxide Formula: Meaning, Structure, and Uses

Suppose you heat another sample of magnesium inpure nitrogento completely convert it to magnesium nitride. Historically, we know it by the name Magnesia Alba the white mineral of magnesia , to distinguish it from magnesia Negra a black mineral that contains what we know as manganese. This supplement is available without a prescription. Using the data above, calculate the mass of oxygen reacted with the sample of magnesium taken, and then click hereto check your answer. Due to this reason, instead of molecular geometry, the crystal lattice structure is studied. Your answer should be between 0. As a result, the formula for Magnesium Oxide is MgO.
Chapter 6 Ionic and Molecular compounds Flashcards

It is used as an antacid and mild laxative and has many nonmedicinal uses. Since magnesium is a Group 2A element, it forms +2 ions: Mg2+. Since oxygen is a Group 6A element, it forms -2 ions: O2-. As Oxygen is more electronegative, it tends to attract valence electrons towards itself whereas, the magnesium atom tends to donate its two valence electrons to form its stable electronic configuration. Solved Example for You Question: Is magnesium oxide stable? It is used as an antacid to treat indigestion, etc. Calculate the number of moles of oxygen atoms contained in 0.
Is Mg2O2 a correct formula for Magnesium Oxide? Why or why not?

The sum of n terms of AP is the sum addition of first n terms of the arithmetic sequence. Here are the steps to follow for using this arithmetic series calculator: First, enter the value of the First Term of the Sequence a1. Na+, most prevalent positive ion in extracellular fluid a. It exists as white, odourless powder. Is magnesium oxide acidic or basic? If you have any queries, drop a comment below, and we will get back to you. Several Live sessions also occur on the channel where the child can ask their doubts and clear their concepts. Such as in case of magnesium oxide, the valency of magnesium is +2 and that of oxygen is -2.
How can I calculate the empirical formula of magnesium oxide?

It decomposes into Magnesium Oxide and water, making it an effective smoke suppressant and fire extinguisher. Its refractive index is 1. Safety and health hazards of magnesium oxide It causes irritation in eyes, and respiratory tract and allergic reaction on skin. Mass of Oxygen Reacted Since the product of your reaction should be puremagnesium oxide containing magnesium and oxygen , and the initial material taken was pure elementalmagnesium, the mass of oxygen reacted should just be thedifferencein these two masses. MgO Explanation: When we write the formula for an ionic compound like magnesium oxide, we take care of the valencies on each of the ion. If they need help, they can use Vedantu's FREE video lectures that are available for everyone on Vedantu's Youtube Channels.
What is the formula for magnesium oxide?

Also, we can also prepare it in laboratories, industries, and research centers. Magnesium provides two electrons to oxygen in an exothermic process, resulting in powdered magnesium oxide MgO. What happens when magnesium oxide is mixed with water? The student can also explore through the list of most important questions that are prepared for the students who have less time and need to score good marks. An arithmetic series is the sum of an arithmetic sequence. Then enter the value of the Common Difference d. At high temperatures, it is a solid that is both physically and chemically stable.