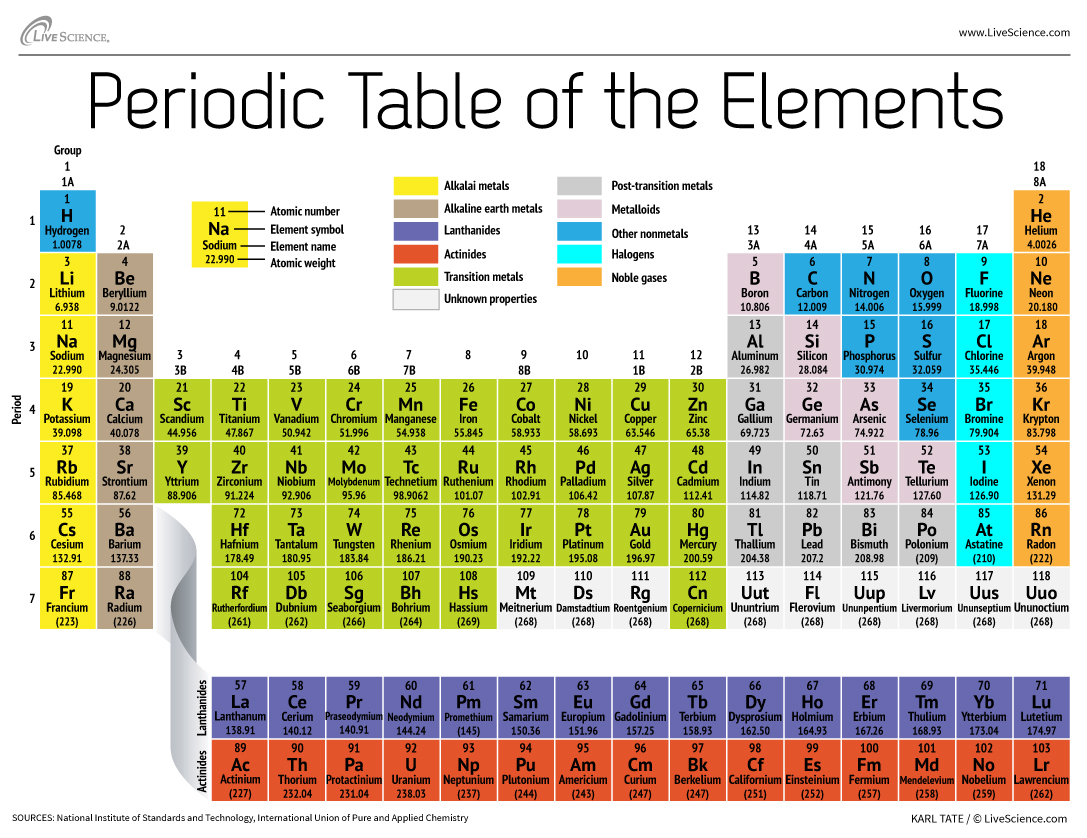
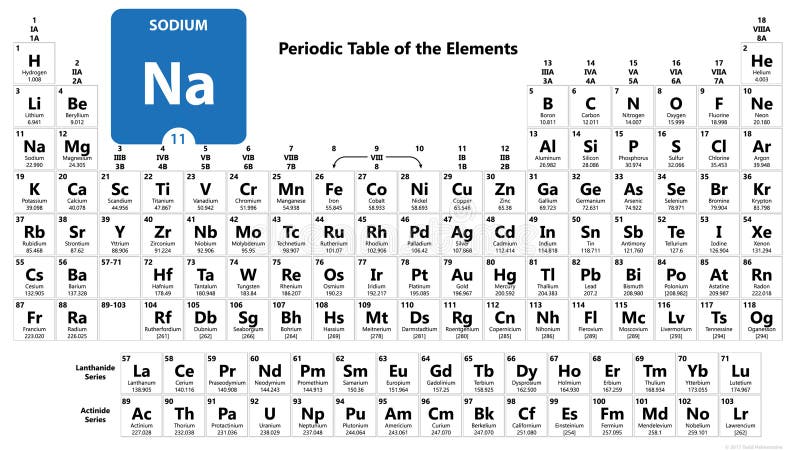
Sodium is a chemical element with the symbol Na and atomic number 11. It is located in the alkali metals group of the periodic table, which consists of elements in the first column on the far left side of the table.
There are several reasons why sodium is located where it is on the periodic table. One reason is its atomic structure. Sodium has a single valence electron, which means it only has one electron in its outermost energy level. This electron is not strongly attracted to the nucleus and is relatively easy to remove, making sodium a highly reactive element.
The alkali metals, including sodium, are known for their high reactivity, which is due to their valence electrons. These elements are prone to losing their valence electrons to form positive ions, or cations. This is because the energy required to remove the valence electron is relatively low, making it easier for them to form chemical bonds with other elements.
Another reason why sodium is located in the alkali metals group is its physical properties. Sodium is a soft, silvery-white metal that is highly malleable and ductile. It has a low melting and boiling point, which makes it easy to melt and vaporize. It is also highly reactive with water, which can cause it to ignite and burn.
In addition to its atomic structure and physical properties, the location of sodium on the periodic table is also influenced by its chemical properties. Sodium is known for its ability to form compounds with a variety of elements, including oxygen, chlorine, and hydrogen. These compounds are important in a wide range of applications, including the production of soap, glass, and various types of chemicals.
Overall, the location of sodium on the periodic table reflects its atomic structure, physical properties, and chemical properties. Its placement in the alkali metals group is due to its high reactivity, low melting and boiling point, and ability to form compounds with other elements.

:max_bytes(150000):strip_icc()/PeriodicTableCrystal-56a12d9b5f9b58b7d0bccfdf.png)