What makes water the universal solvent. Water, the Universal Solvent 2022-10-31
What makes water the universal solvent
Rating:
9,2/10
124
reviews
Water is a universal solvent because it has a number of unique chemical and physical properties that allow it to dissolve a wide range of substances.
One of the key factors that makes water a universal solvent is its polarity. Water molecules have a positive charge at one end and a negative charge at the other, creating a polar molecule. This means that water molecules are attracted to ions and other polar molecules, allowing them to dissolve and interact with these substances.
In addition to its polarity, water has a high surface tension and is a good solvent for many organic molecules. These properties allow it to dissolve and interact with a wide range of substances, including sugars, salts, and amino acids.
Another important factor that contributes to water's ability to dissolve a wide range of substances is its ability to form hydrogen bonds. These bonds are relatively weak, but they are numerous and are easily formed between water molecules and other polar molecules. This allows water to dissolve and interact with a wide range of substances, including many organic molecules.
In summary, water's polarity, high surface tension, and ability to form hydrogen bonds make it a universal solvent. These properties allow it to dissolve and interact with a wide range of substances, making it an essential component of many chemical reactions and biological processes.
What property makes water the universal solvent?

This helps water dissociate ionic compounds into their positive and negative ions. Raising the temperature typically increases the effectiveness of a solvent because it increases the kinetic energy of particles. Solution — A liquid that is a homogenous mixture of two or more substances. It can make solutions out of most compounds, save a few organic materials. Salt remains in solution in water indefinitely unless a process like desalinization is employed.
Next
Water, the Universal Solvent

Many aquatic species rely on oxygen in the water to survive. A substance that dissolves all others cannot be stored because the container would be dissolved. Sugar is much more soluble in water than is salt. This explains why river fish cannot survive in a home aquarium because there is insufficient oxygen. The ionic bond is a strong chemical bond, but the action of all the water molecules is sufficient to pull the sodium and chlorine atoms apart.
Next
Universal Solvent

Why water is termed as universal solvent,discuss in details? Due to the above-mentioned factors, water is capable to dissolve ionic lattices. Sugar dissolves in water because energy is given off when the slightly polar sucrose molecules form intermolecular bonds with the polar water molecules. Water is called the universal solvent because it dissolves more substances than any other solvent although not all. What types of solutes can water dissolve? He described mixing sal alkali with olive oil to produce sweet oil, likely glycerol. It can dissolve solids, liquids and gases. Not only we manufacture lab items, we export to international board affiliation requirements for schools by providing affiliation packages to schools. Why does water act as a biological solvent? This is important to every living thing on earth.
Next
Why water is a universal solvent?

Water is an ideal biologic solvent because of its unique chemical structure. Solvent A solvent is a substance in which a solute dissolves and creates a solution. Because of its polarity and ability to form hydrogen bonds, water makes an excellent solvent, meaning that it can dissolve many different kinds of molecules. Water is called the universal solvent because it Why Water Is Called the Universal Solvent Water dissolves more chemicals than any other solvent because its polar nature gives each molecule a hydophobic water-fearing and hydrophilic water-loving side. Also, nonpolar molecules don't dissolve very well in water, including many organic compounds, such as fats and waxes. Solvents are usually liquids, although they can also be solids, gases, or supercritical fluids.
Next
What makes water the universal solvent?
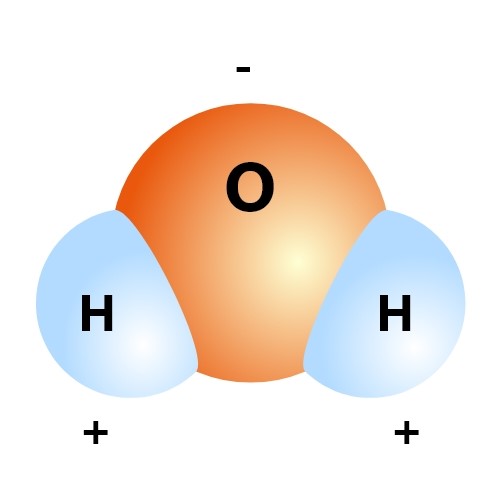
If the attraction is high between the oppositely charged ions in a compound, then the solubility will be low. What does that mean? It also depicts how a charge, such as on an ion Na or Cl, for example can interact with a water molecule. Which solvent dissolves the sugar most quickly? Water dissolves sugar, salt, and other flavourings. A single atom of oxygen forms a connection with two hydrogen atoms. Nonpolar solvents dissolve nonpolar molecules such as fats and other organic compounds. Farrar, Straus, and Giroux.
Next
Why is water considered the universal solvent?

High Specific Heat The amount of energy needed to raise the temperature of one gram of water by 1 degree Celsius is its specific heat, and at one calorie per gram, it is much higher than the specific heat of most liquids. We hope this detailed article on Water as a Solvent proves helpful to you in your preparations. Â Some demineralized water from Labkafe, very pure This leads us to comment on one thing. There are several nonpolar organic solvents. Because of its polarity and ability to form hydrogen bonds, water makes an excellent solvent, meaning that it can dissolve many different kinds of molecules. It does not dissolve nonpolar molecules, including organic compounds such as fats and oils.
Next
What quality makes water the universal solvent?

Is water a universal solvent? What quality makes water the universal solvent? If a lot of salt is mixed with water, it won't all dissolve. After it was discovered, people found a variety of interesting uses for it, including as an aftershave lotion, and over time they recognized as an effective solvent and degreaser. As they move faster, they come into contact with the sugar more often, causing it to dissolve faster. Water-based solutions, such as blood aid in the transportation of molecules to their proper sites. Some alchemists, including Philalethes, got around this argument by claiming alkahest would only dissolve material down to its elements. The oxygen atom, however, is somewhat greedy and pulls the shared electrons closer to itself.
Next
Why Is Water the Universal Solvent?

The hydrogen side of each water H 2 O molecule carries a slight positive electric charge, while the oxygen side carries a slight negative electric charge. What Is a Solvent? Some colours are water-soluble. Other molecules, such as sucrose or sugar, aren't torn into ions, but disperse evenly in water. Not all substances are soluble in water. What properties of water make it useful as a solvent? For example, most of the hydroxides exhibit low solubility in water.
Next
Universal Solvent Definition

More kinetic energy results in more interaction between particles, so dissolving occurs more quickly. A true universal solvent does not exist. The alchemists sought such a compound, which they called alkahest. In this compound, there are two hydrogen atoms and one oxygen atom bound by a covalent bond. Because water molecule is comprised of two hydrogen atoms and one oxygen atom , this makes the solvent polar.
Next








