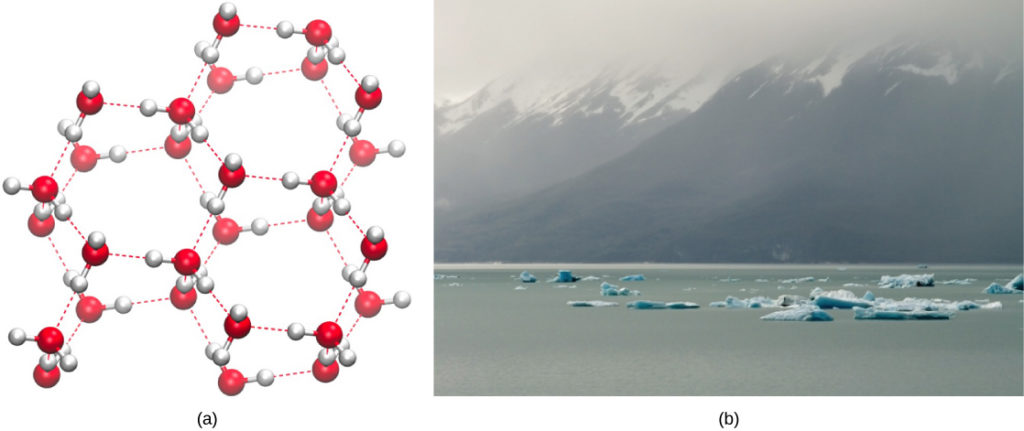
Water is a unique and essential molecule that plays a vital role in supporting life on Earth. There are several properties of water that contribute to its ability to support life.
First, water is a universal solvent. This means that it can dissolve a wide variety of substances, including salts, sugars, and other organic molecules. The ability of water to dissolve and transport these substances is crucial for the functioning of living cells and the maintenance of homeostasis within the body. For example, water helps to transport nutrients and oxygen to cells, and it helps to remove waste products from the body.
Second, water has a high heat capacity. This means that it requires a lot of energy to raise its temperature, and it can absorb or release a large amount of heat without changing its own temperature. This property allows water to regulate the temperature of living organisms and their environments. For example, when an organism produces heat, water in the body can absorb that heat and prevent the organism's temperature from rising too high. Similarly, when an organism's surroundings are too cold, water in the body can release heat to keep the organism warm.
Finally, water has a high surface tension. This means that it forms a strong, cohesive film on surfaces, which allows it to hold its shape when it is in contact with air. This property is important for the buoyancy of aquatic organisms and the ability of plants to transport water from their roots to their leaves. It is also responsible for the ability of water to form droplets and beads on hydrophobic surfaces, which is important for the functioning of the water cycle and the maintenance of moisture in the environment.
In conclusion, the properties of water that make it a universal solvent, a high heat capacity, and a high surface tension are all essential for the support of life on Earth. These properties allow water to transport substances, regulate temperature, and maintain hydration, all of which are necessary for the functioning of living cells and the maintenance of a stable environment.
How do the properties of water make life on earth possible?

Finally, the high specific heat of water makes it resistant to temperature change, allowing life forms to maintain relatively constant internal temperatures. What is special about water and density? The main properties of water are its polarity, cohesion, adhesion, surface tension, high specific heat, and evaporative cooling. Why is water less dense at 4°C? This is the currently selected item. What are the 4 properties of water quizlet? Properties of Water and its Importance to Life. What are the 7 properties of water and its importance? Water protects organisms from rapid temperature changes and helps them to maintain their normal internal temperatue. Water has several properties that make it unique amongst compounds and make it possible for all forms of known life to function. Water can ionize: A small amount of water spontaneously dissociated into hydrogen ion H + and hydroxyl ion OH — which depends on temperature.
What are the 4 properties of water that allow life?

What are the properties of water that make life possible? Because of hydrogen bonding, water is a liquid at room temperatures suitable for life, it boils at 100 degree centigrade and freezes at 0 centigrade. What are the 4 properties of water that make it so unique? Water in nature contains minerals, gasses, salts, and even pesticides and bacteria, some of which are dissolved. Life-forms use water to carry nutrients around the body and to take away waste. Water is highly cohesive and adhesive: Because of hydrogen bonds, water molecules develop strong intermolecular attraction between them. What is the importance of water in Earth? What are the unique properties of water that make it so critical to life? The unique physical properties, including a high heat of vaporization, strong surface tension, high specific heat, and nearly universal solvent properties of water are also due to hydrogen bonding.
What are three reasons water is important to life on Earth? Water is an extremely potent solvent due to its characteristic of high polarity. The five main properties that will be discussed in this article are its attraction to polar molecules, its high specific heat, the high heat of vaporization, the lower density of ice, and its high polarity. The main properties of water are its polarity, cohesion, adhesion, surface tension, high specific heat, and evaporative cooling. Surface tension protects marine ecosystems. The many hydrogen bonds that link water molecules help water absorb heat without a great change in temperature. The unique properties characteristics of water make life possible on earth. Why is water so important to living things? When water form hydrogen bonds with other substance, the attraction is called adhesion.