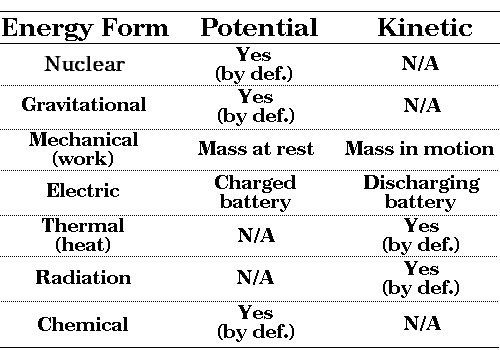
Thermal energy and kinetic energy are two forms of energy that are often discussed in the context of physics and thermodynamics. Thermal energy is the energy that is associated with the temperature of a system, while kinetic energy is the energy that an object possesses due to its motion. Both of these forms of energy are important in a variety of applications and have significant impacts on the behavior of systems.
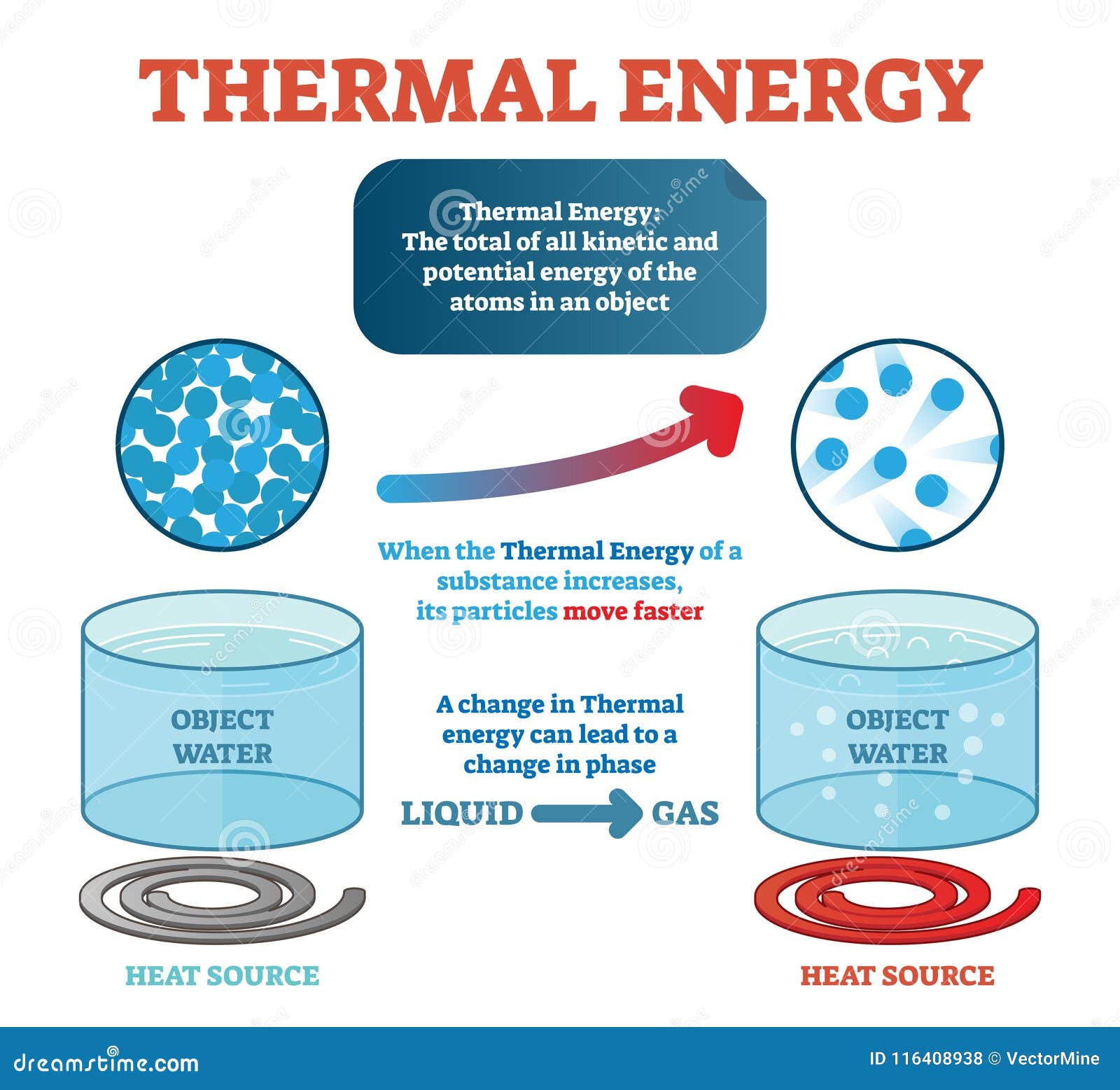
Thermal energy is a measure of the internal energy of a system due to the movement and interactions of the particles that make up the system. It is a form of energy that is associated with the temperature of a system and can be transferred between systems through various means, such as conduction, convection, and radiation. Thermal energy is an important factor in a wide range of applications, including the functioning of engines, the performance of materials, and the efficiency of heating and cooling systems.
One of the key properties of thermal energy is that it is a form of energy that is related to the temperature of a system. The more thermal energy a system has, the higher its temperature will be. This relationship between thermal energy and temperature is described by the laws of thermodynamics, which provide a framework for understanding the behavior of systems in terms of energy and heat.
Kinetic energy, on the other hand, is the energy that an object possesses due to its motion. It is a measure of the energy that is required to accelerate an object to a given velocity, and is related to the mass of the object and its velocity. Kinetic energy is an important factor in a wide range of applications, including the functioning of machines, the performance of athletes, and the energy required to perform various tasks.
One of the key properties of kinetic energy is that it is a form of energy that is related to the motion of an object. The more kinetic energy an object has, the faster it will be moving. This relationship between kinetic energy and velocity is described by the laws of motion, which provide a framework for understanding the behavior of objects in terms of force and motion.
Thermal energy and kinetic energy are both important forms of energy that play key roles in a wide range of applications. Understanding these forms of energy and how they interact with each other is crucial for developing a deep understanding of the physical world and the behavior of systems.