Isopentyl acetate, also known as banana oil, is an ester with the chemical formula CH3COO(CH2)3CH(CH3)2. It is a colorless liquid with a sweet, fruity odor similar to that of bananas. Isopentyl acetate is used in the production of flavors and fragrances, and is also used as a solvent in the production of resins and plastics.
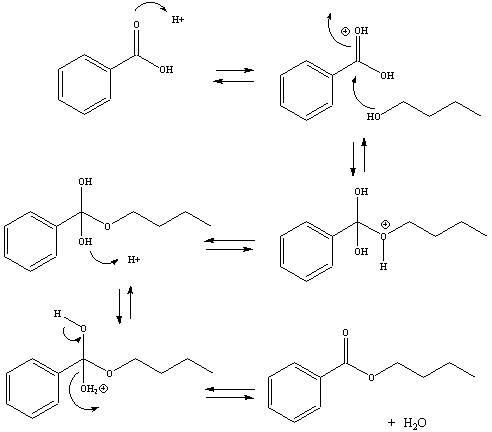
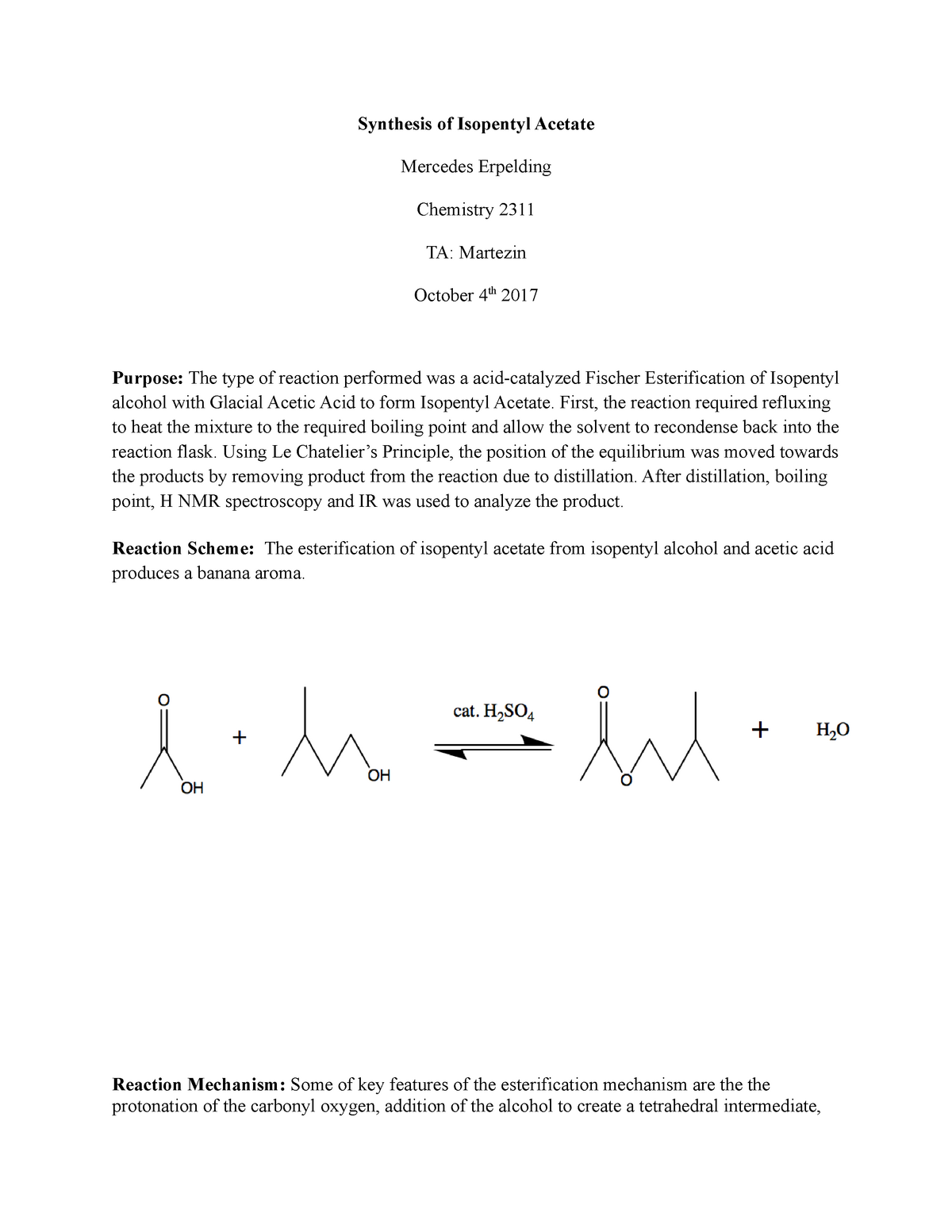
The synthesis of isopentyl acetate involves the reaction of isopentanol with acetic acid in the presence of an acid catalyst. The reaction is known as an esterification reaction, and it occurs through the formation of a covalent bond between the carboxyl group of the acetic acid and the hydroxyl group of the isopentanol.
The synthesis of isopentyl acetate can be carried out using either a batch or a continuous process. In the batch process, the reactants are mixed together in a reaction vessel and allowed to react under carefully controlled conditions. The continuous process involves the continuous flow of the reactants through a series of reaction vessels, where the reaction takes place.
The reaction conditions for the synthesis of isopentyl acetate are critical in order to achieve a high yield of the desired product. The temperature and pressure of the reaction must be carefully controlled to ensure that the reaction occurs at an optimal rate. The acid catalyst is also important in the reaction, as it helps to increase the rate of the reaction by lowering the activation energy.
In addition to the reactants and catalyst, a small amount of water is also typically added to the reaction mixture to help remove the heat of reaction and to promote the formation of the desired product. The water is removed from the reaction mixture as the reaction proceeds, and the final product is isolated by distillation.
Overall, the synthesis of isopentyl acetate is a useful and widely employed process that is used in the production of flavors, fragrances, and various industrial materials. It involves the use of chemical reactions to synthesize a desired product, and requires careful control of reaction conditions in order to achieve a high yield of the desired product.