Iron oxalate is a coordination compound that consists of iron ions and oxalate ions bonded together. It is often synthesized through a process known as precipitation.
To synthesize iron oxalate, you will need the following materials:
- Iron(II) chloride (FeCl2)
- Sodium oxalate (Na2C2O4)
- Water
- Stirring rod
- Beaker or flask
- Filter paper
Here is the synthesis procedure:
In a beaker or flask, dissolve a measured amount of iron(II) chloride in a small amount of water.
Add an excess of sodium oxalate to the iron(II) chloride solution. The molar ratio of iron(II) chloride to sodium oxalate should be 1:2.
Stir the mixture until the sodium oxalate is completely dissolved.
Allow the mixture to stand undisturbed for several hours or overnight.
After the desired amount of time has passed, the iron oxalate complex will begin to precipitate out of the solution.
Filter the mixture using filter paper to separate the solid iron oxalate from the liquid.
Rinse the iron oxalate with a small amount of water to remove any impurities.
Allow the iron oxalate to dry completely before weighing and storing it.
Iron oxalate can also be synthesized using other methods, such as the solvent extraction method or the co-precipitation method. However, the precipitation method is the most commonly used method due to its simplicity and ease of execution.
Iron oxalate has a number of potential applications, including its use as a catalyst in the production of chemicals and as a pigment in the manufacturing of dyes and paints. It is also used in the preparation of pharmaceuticals and in the treatment of iron deficiency anemia.
In conclusion, the synthesis of iron oxalate complex involves the precipitation of the compound from an iron(II) chloride and sodium oxalate solution. This process is relatively simple and can be easily scaled up for large-scale production. Iron oxalate has a range of applications, making it a useful and versatile compound.
LAB 7

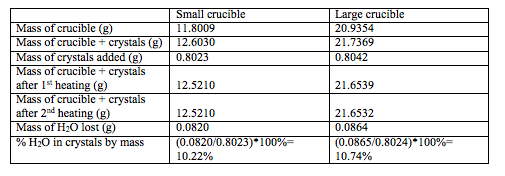
Once it has fully dissolved, the cold iron oxalate solution is added to the hot potassium oxalate solution. The oxidation of the oxalate anion which is an organic chelating agent, does not take place very easily. Also the purpose of the experiment is to determine the actual, theoretical, and percent yields of product, and characterize the final compound by determining the number of waters of hydration by gravimetric analysis…. Obtain in a clean 50 mL beaker 8. Molecules exist in the unstable state by absorption of light and this reaction induces the electronic reorganization. While the experiment was performed, the filtering crucible was set-aside in a desiccator to cool and stay dry. Assuming that all of the Fe originally in FeCl3 ends up in the product, KxFe C2O4 y.
Synthesis Of An Iron ( IIi )

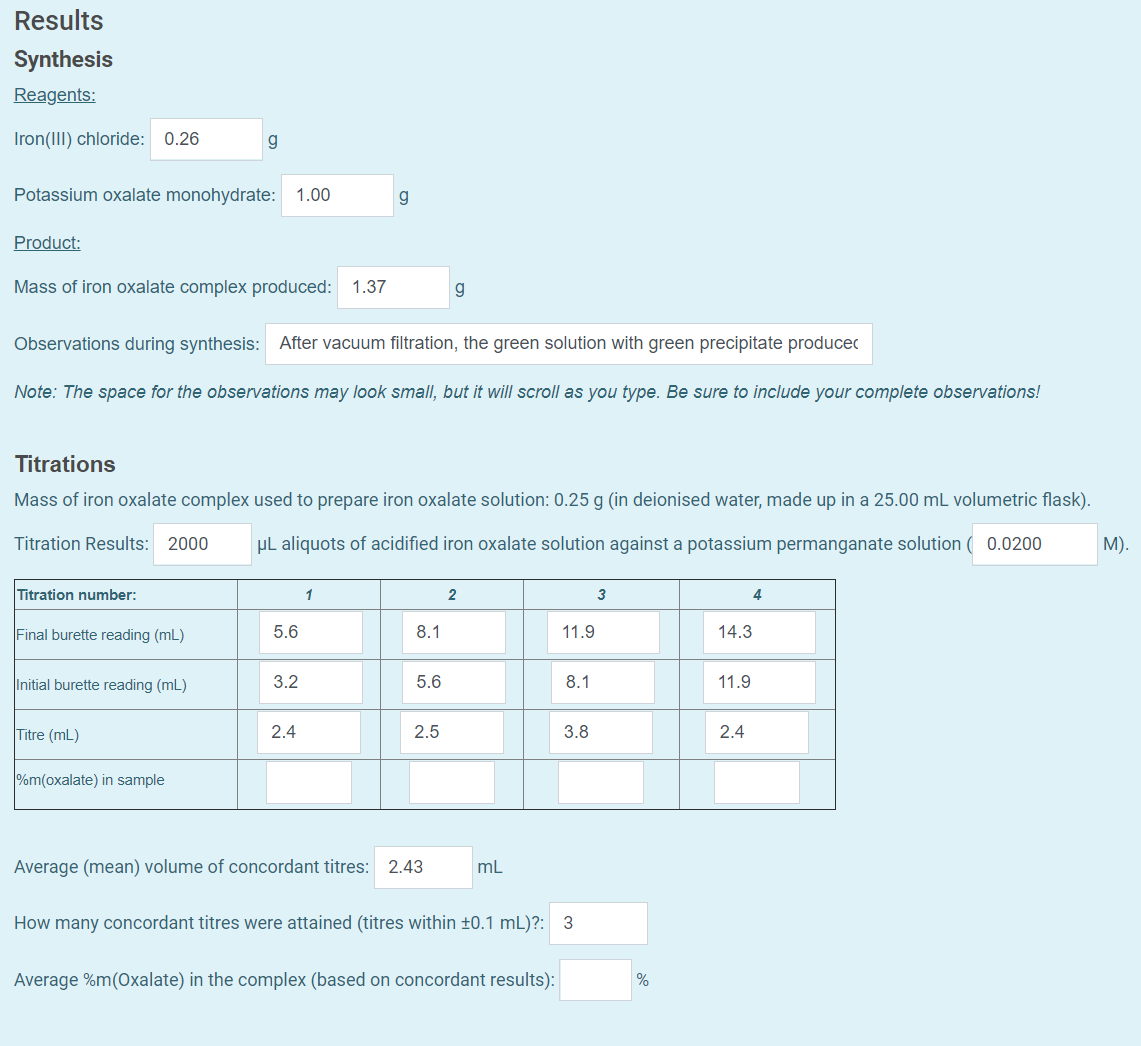
Probably some product is lost in crystallizing the separating crystals from supernatant liquid, etc. When titrating the potassium permanganate with the potassium trioxalatoferrate III trihydrate salt, a faint pink colour is observed when the stoichiometric point has been reached thus titration would be completed. This was filtered through a glass wool and the residual was washed with 2M sulphuric acid. So, molecules are easily decomposed or they can be combined with other compounds Andreas Luz, J. Experiment C: The analysis of the products for iron and oxalate Iron II oxalate 0.
Experiment 4: Synthesis and Analysis of an Iron(III)

The precipitation is added by leaving the mixture overnight so that the salt would precipitate. The second crop of recrystallized product is generally less pure that the first. In the first part the oxalate and iron II are both oxidized to Iron III and carbon dioxide. Bidentate ligands donate two pairs of electrons such as the oxalate ligands which can bind at two sites with the metal ion, thus a coordination number of three ligands around one metal ion. This was dried,weighed and the product kept in the dark.
(DOC) Synthesis and Analysis of Iron (III) Oxalate Complex
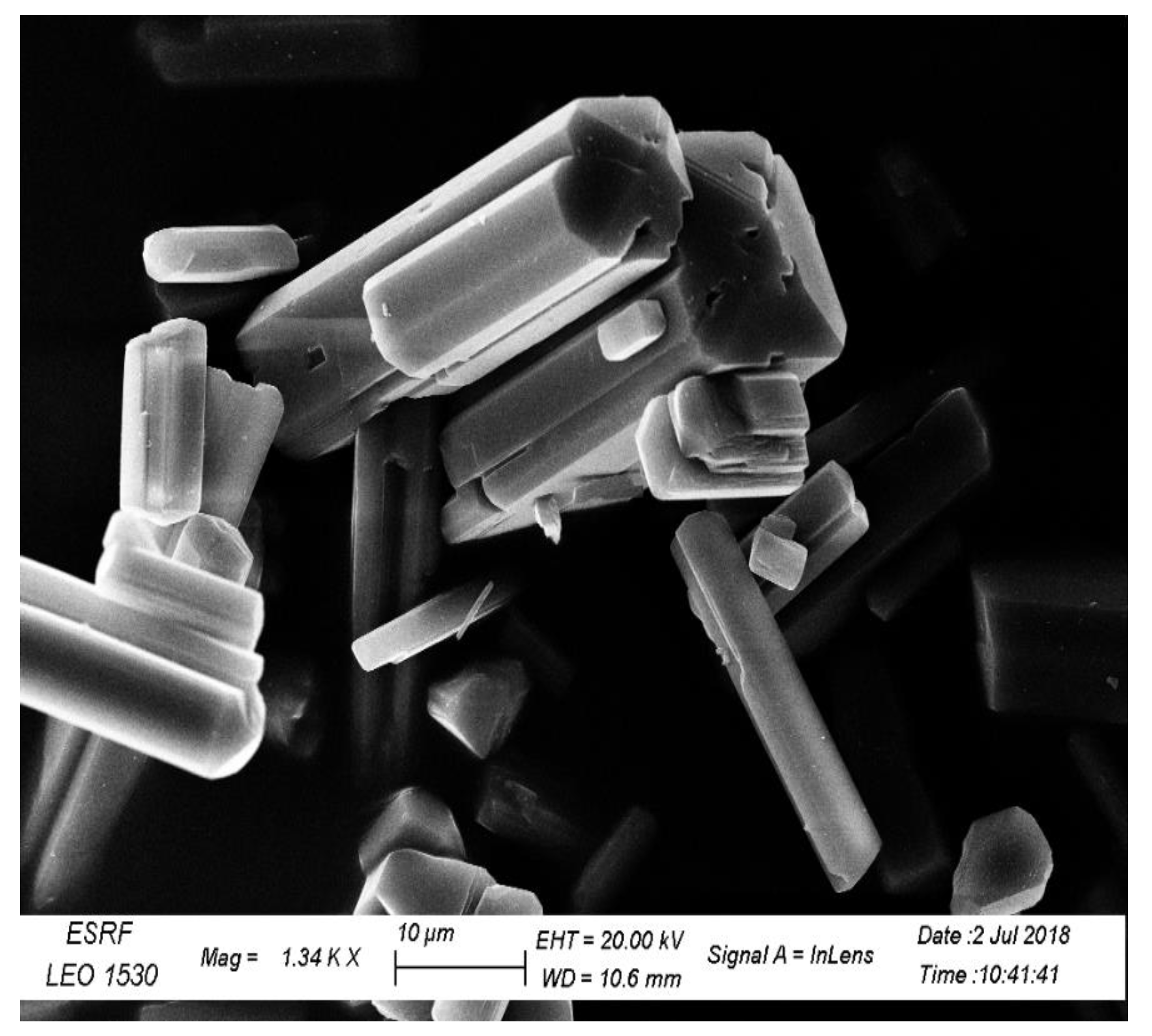
The questions that I need to answer: 4 What is the shape of the crystals of the product? A percentage yield of 11. Temperature control is very crucial in this step due to the fact at high temperatures, hydrogen peroxide can decompose and thus would not be able to oxidise the iron II to iron III required to prepare the Potassium trioxalatoferrate III trihydrate complex. Observations: Ferrous II oxalate had a yellow precipitate and at the end a yellow powder was obtained. The number of millimoles present in 100g of complex were found to be, for unknown samples 3-5: 215. METHODS: In the first part of the experiment, the synthesis of green crystals was prepared first by mixing hydrated salt with deionized water and sulfuric acid, which gave 2 + ¿ Fe ¿ solution. Apparatus: Two 50 mL beakers, centigram balance, crucible tongs, 250 mL beaker, watch glass, vacuum filtration apparatus with Buchner funnel, small brown bottle or bottle with aluminum foil. The percentages of iron and oxalate in the complex were determined and this was compared to the theoretical value.
Synthesis and analysis of an iron oxalate webapi.bu.edu

From simple essay plans, through to full dissertations, you can guarantee we have a service perfectly matched to your needs. Permanganate solution when allowed to stand in burette can undergo partial decomposition to MnO2. During the addition of oxalic acid, the solution was maintained near the boiling point. Get Help With Your Essay If you need assistance with writing your essay, our professional essay writing service is here to help! Copy to Clipboard Reference Copied to Clipboard. Finally wash the crystals twice with 5 mL portions of acetone. The final product may be given the formula KxFe C2O4 y.
_Oxalate_Structural_Formula_V1.svg/1200px-Copper_(II)_Oxalate_Structural_Formula_V1.svg.png)

-oxalate-sample.jpg)