Standardizing a solution of sodium hydroxide involves determining the precise concentration of the solution. This is important because sodium hydroxide is a strong base that is commonly used in various chemical reactions and it is important to know the exact concentration in order to accurately measure out the correct amount for a particular reaction.
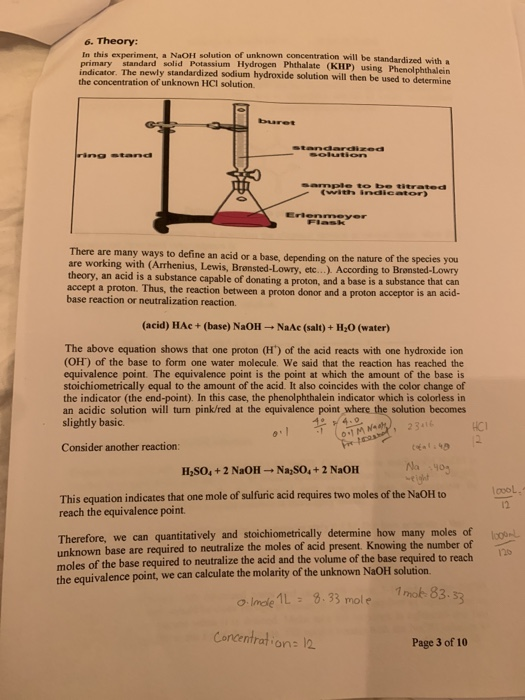
To standardize a solution of sodium hydroxide, a primary standard acid is needed. A primary standard acid is a highly pure and stable acid that can be accurately weighed and dissolved in water to create a known concentration. A common primary standard acid used in this process is potassium hydrogen phthalate (KHP).
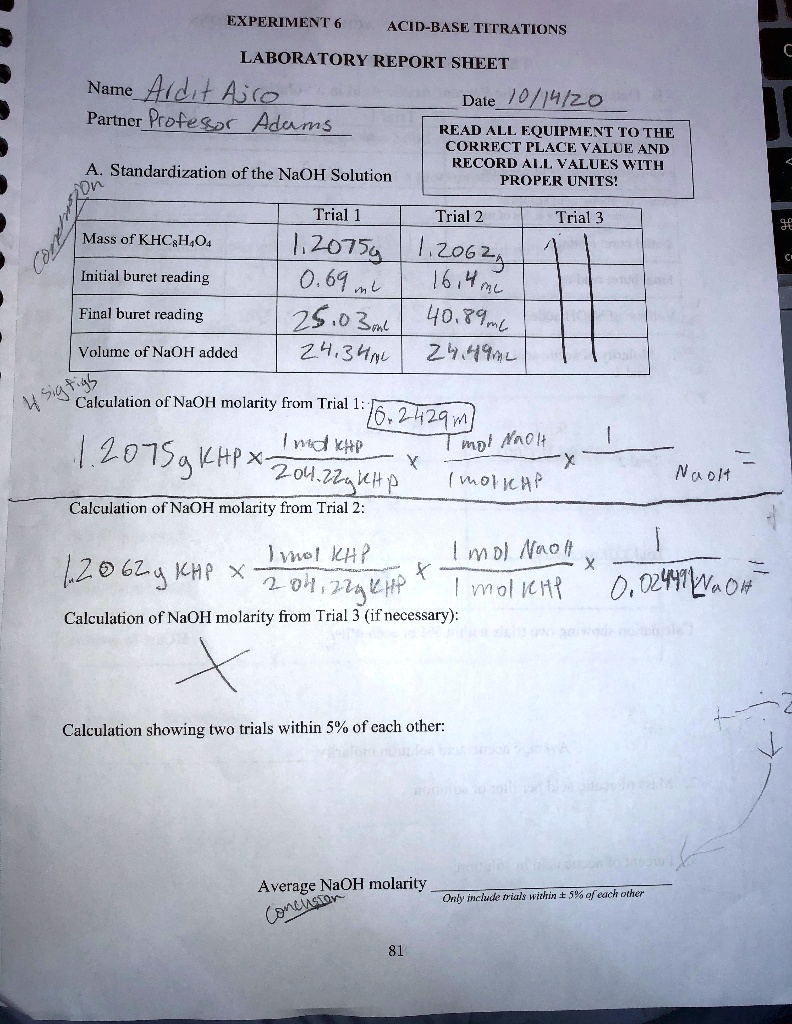
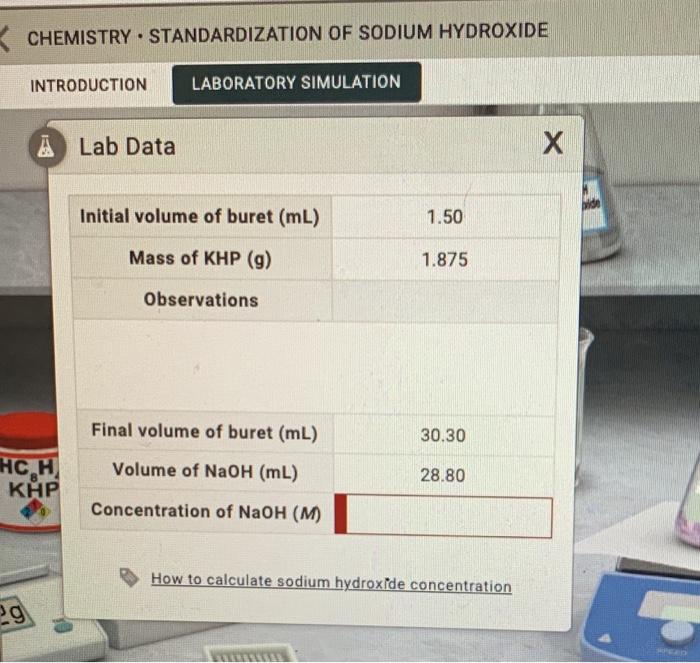
To standardize the sodium hydroxide solution, a sample of the KHP is accurately weighed and dissolved in a small amount of water to create a known concentration. Then, a measured volume of the KHP solution is placed in a conical flask and an indicator, such as phenolphthalein, is added. The sodium hydroxide solution is then added to the KHP solution dropwise until the endpoint of the reaction is reached, as indicated by a change in the color of the indicator.
The endpoint is determined by the point at which the acid and base have completely neutralized each other, resulting in a pH of 7 (neutral). The amount of sodium hydroxide solution used to reach the endpoint is recorded and used to calculate the concentration of the sodium hydroxide solution.
The concentration of the sodium hydroxide solution is calculated using the following equation:
Concentration (M) = (Volume of sodium hydroxide used (mL) * Normality of KHP) / Mass of KHP used (g)
The normality of the KHP solution is determined by the concentration of the acid and the number of hydrogen ions it can donate in a reaction.
Once the concentration of the sodium hydroxide solution has been determined, it can be accurately used in chemical reactions and other applications. It is important to note that the process of standardizing a solution of sodium hydroxide should be repeated periodically to ensure the accuracy and precision of the solution.
In conclusion, standardizing a solution of sodium hydroxide is an important process that involves determining the precise concentration of the solution using a primary standard acid. This process ensures that the correct amount of sodium hydroxide can be accurately measured and used in chemical reactions and other applications.




.jpg)