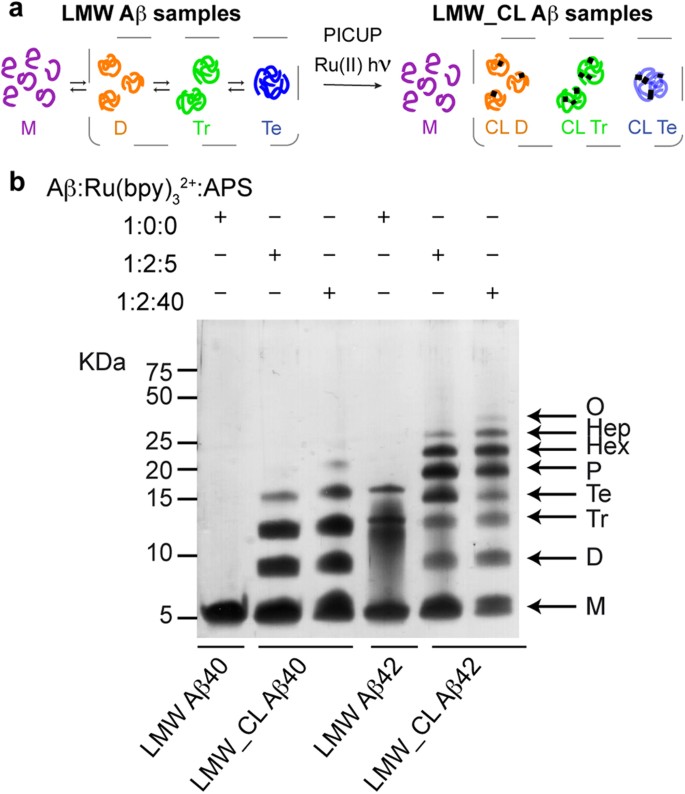
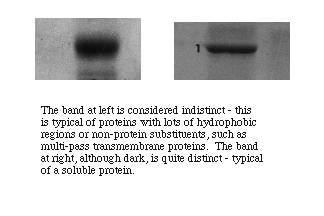
SDS PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) is a commonly used technique in molecular biology laboratories for separating and analyzing proteins based on their size and charge. In this experiment, a protein sample is mixed with SDS, a detergent that coats the protein and gives it a uniform negative charge. The protein-SDS mixture is then loaded onto a polyacrylamide gel, which is placed in a special apparatus and subjected to an electric current. As the protein moves through the gel, it separates into individual bands based on its size, with the smaller proteins moving faster through the gel than the larger ones.
To begin the experiment, the necessary materials and equipment are assembled, including a protein sample, SDS, polyacrylamide gel, a gel apparatus, and a power supply. The protein sample is then mixed with SDS according to the manufacturer's instructions, taking care to ensure that the SDS is evenly distributed throughout the sample.
Next, the polyacrylamide gel is prepared according to the manufacturer's instructions, taking care to ensure that the gel is properly formed and free of defects. Once the gel is prepared, it is placed in the gel apparatus and the protein-SDS mixture is carefully loaded onto the gel. The apparatus is then connected to the power supply, and the electric current is turned on.
As the protein moves through the gel, it separates into individual bands based on its size. These bands can be visualized using a special stain or by placing the gel under UV light, which causes the protein to fluoresce.
The results of the SDS PAGE experiment can be analyzed and interpreted in a number of ways. The size of the protein bands can be measured and compared to known standards, allowing researchers to determine the size of the proteins in the sample. In addition, the presence or absence of certain protein bands can provide information about the purity of the sample, as well as any post-translational modifications that may have occurred.
Overall, SDS PAGE is a powerful tool for separating and analyzing proteins, and is an important technique in many areas of molecular biology research. It is a reliable and reproducible method that allows researchers to gain valuable insights into the structure and function of proteins, which is critical for understanding biological processes at the molecular level.