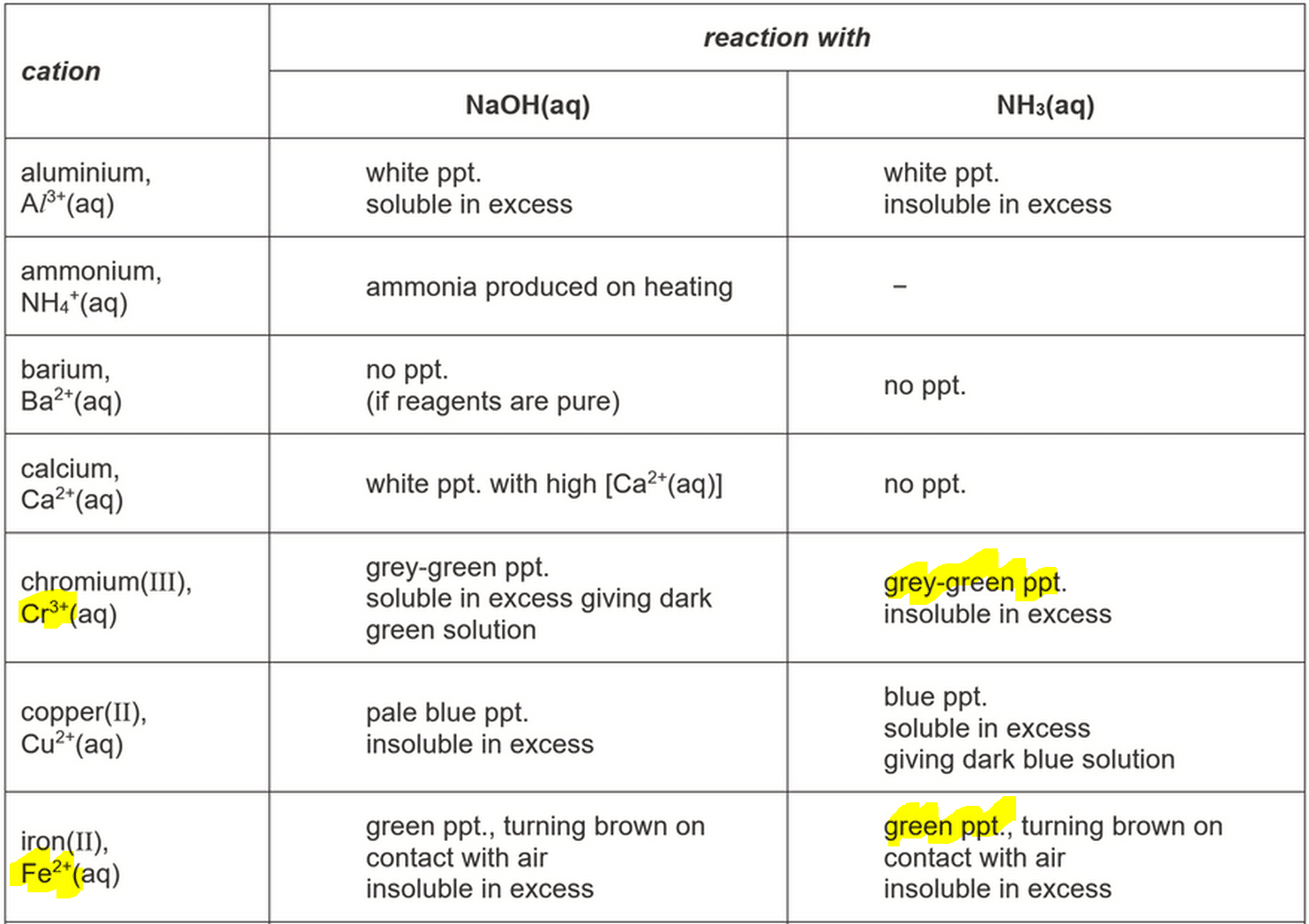
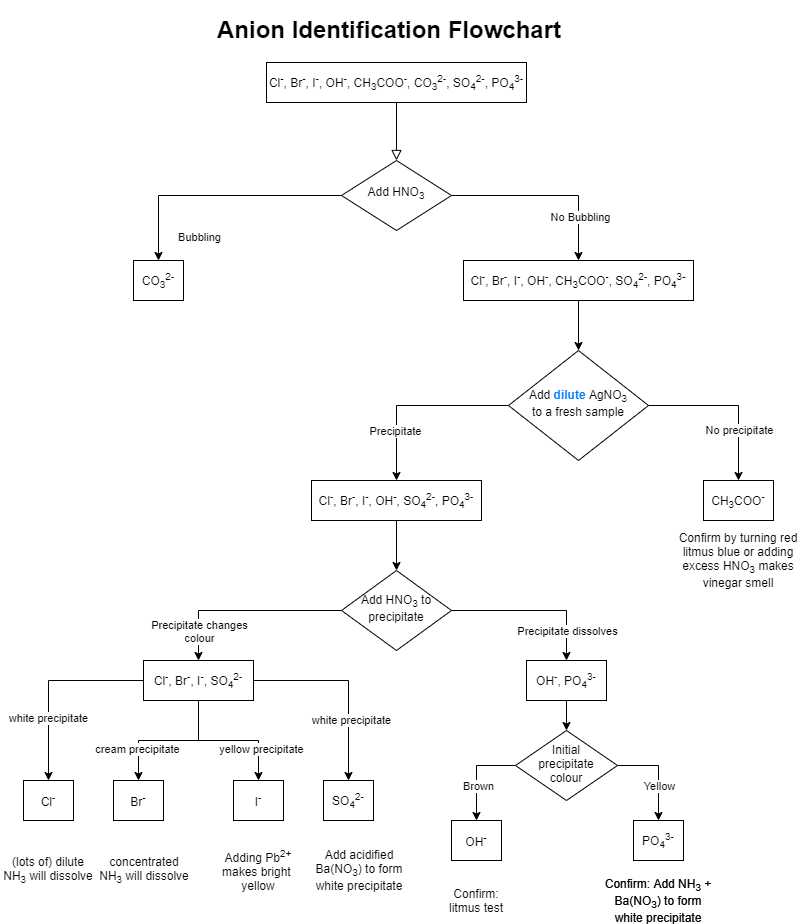
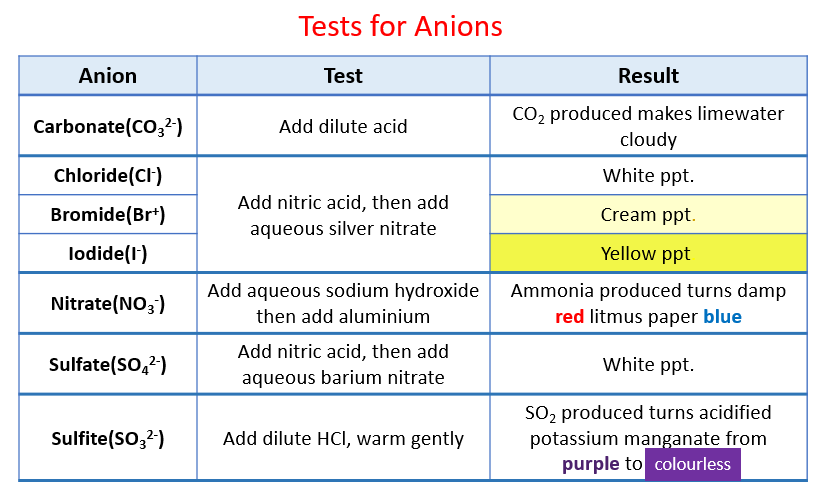
Qualitative inorganic analysis is a branch of chemistry that deals with the identification of elements present in a sample. One common type of qualitative inorganic analysis is the analysis of anions, which are negatively charged ions that are found in compounds. In this essay, we will discuss the methods and techniques used in the qualitative inorganic analysis of anions.
There are several methods that can be used to perform the qualitative inorganic analysis of anions. One common method is the use of precipitate reactions, in which a reagent is added to the sample, causing a precipitate to form. The presence of a precipitate indicates the presence of a particular anion in the sample. For example, the addition of a silver nitrate solution to a sample containing chloride ions will result in the formation of silver chloride, which can be identified by its white precipitate.
Another method used in the qualitative inorganic analysis of anions is the use of flame tests. In this method, a sample is placed in a flame, and the resulting color of the flame is observed. Each anion produces a unique flame color, allowing the anion to be identified. For example, the flame produced by a sample containing sodium ions will be yellow, while the flame produced by a sample containing potassium ions will be purple.
A third method used in the qualitative inorganic analysis of anions is the use of spectroscopy techniques. These techniques involve the use of instruments that can measure the absorption or emission of light by a sample. Different anions absorb or emit light at different wavelengths, allowing the anion to be identified. Some common spectroscopy techniques used in the qualitative inorganic analysis of anions include infrared spectroscopy, ultraviolet-visible spectroscopy, and nuclear magnetic resonance spectroscopy.
Overall, the qualitative inorganic analysis of anions is an important field of chemistry that allows us to identify the elements present in a sample. By using techniques such as precipitate reactions, flame tests, and spectroscopy, we can accurately determine the presence and quantity of anions in a sample. This information is useful in a variety of fields, including medicine, environmental science, and materials science.