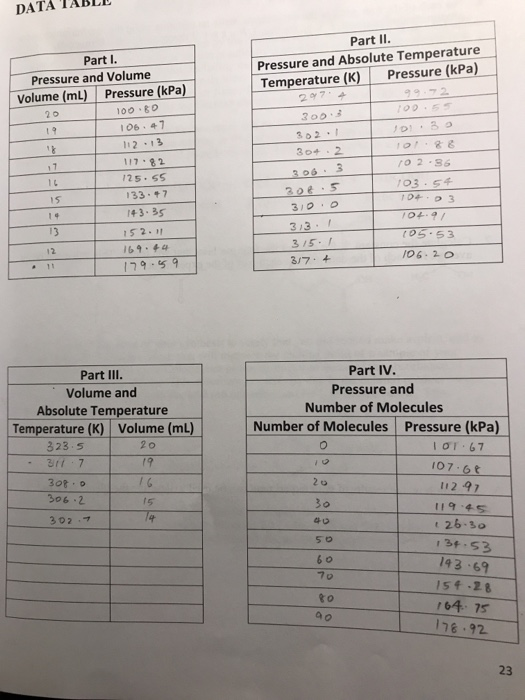
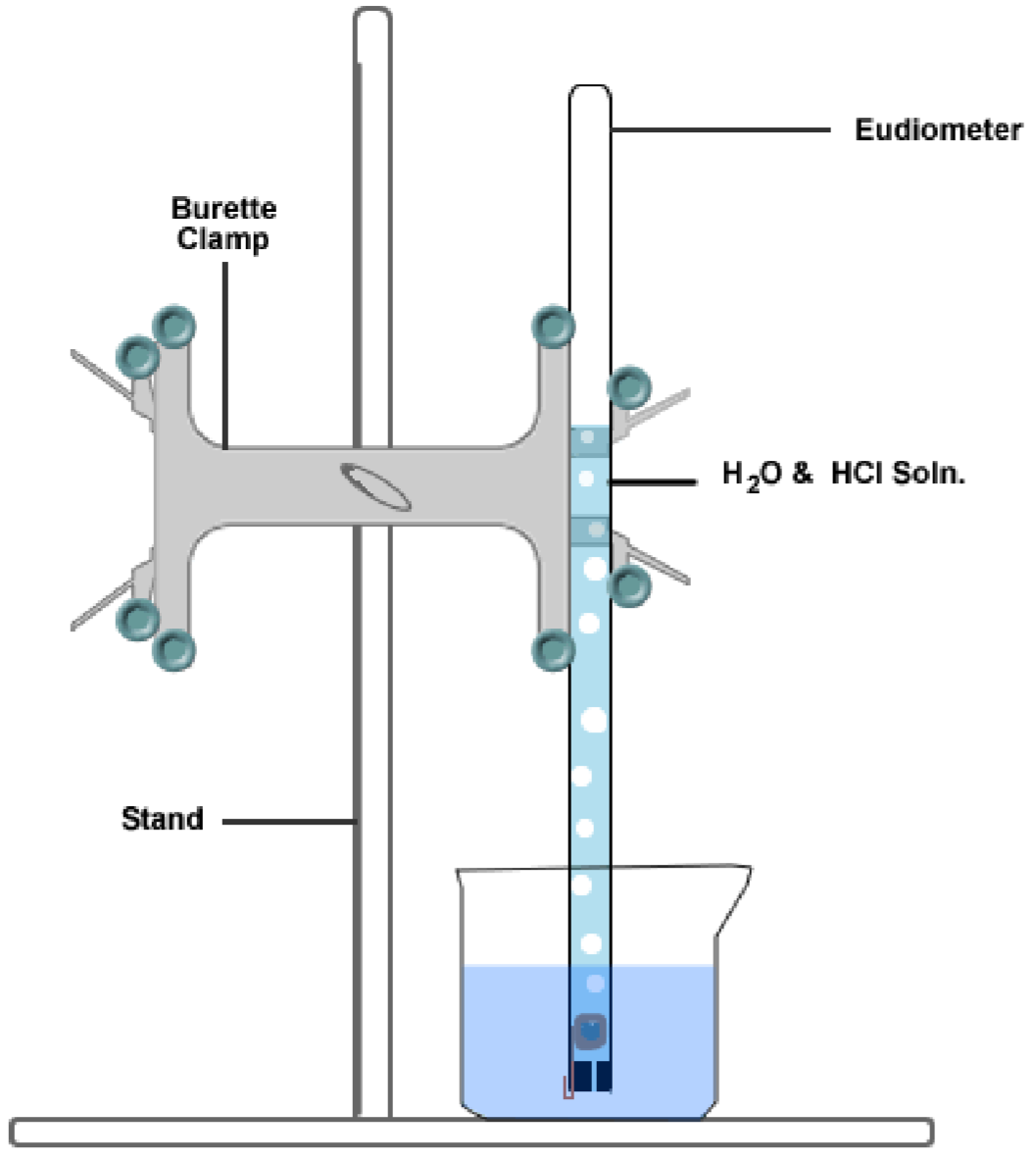
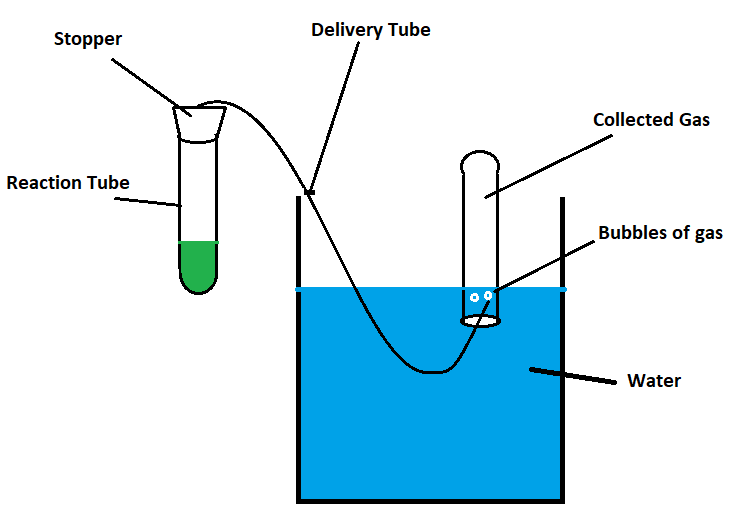
Gases have several unique properties that make them distinct from other states of matter. In a lab setting, these properties can be observed and measured through various experiments and techniques.
One property of gases is their compressibility. Gases are able to be compressed, meaning that they can be squeezed into a smaller volume. This can be demonstrated through the use of a pressure gauge, which measures the pressure of a gas in a container. When the volume of the container is decreased, the pressure of the gas increases. Conversely, when the volume of the container is increased, the pressure of the gas decreases.
Another property of gases is their expansion. Gases are able to expand to fill the space available to them. This can be demonstrated through the use of a balloon. When a balloon is filled with a gas, it expands to fill the volume of the balloon. Similarly, if a gas is released into a container, it will expand to fill the entire container.
The buoyancy of gases is another notable property. Gases are less dense than liquids and solids, and as a result, they tend to rise to the top of a container. This can be demonstrated through the use of a flask filled with a gas and a liquid. The gas will rise to the top of the flask, while the liquid will remain at the bottom.
Gases also have low viscosity, meaning they are able to flow easily. This can be demonstrated through the use of a gas flow meter, which measures the flow rate of a gas through a pipe or tubing. The flow rate of a gas is typically much higher than that of a liquid, due to the low viscosity of gases.
Finally, gases are able to mix completely with other gases. This can be demonstrated through the use of a container with multiple gases. When the gases are released into the container, they will mix evenly and uniformly throughout the container.
Overall, the properties of gases make them unique and distinct from other states of matter. Through various experiments and techniques, these properties can be observed and measured in a lab setting.