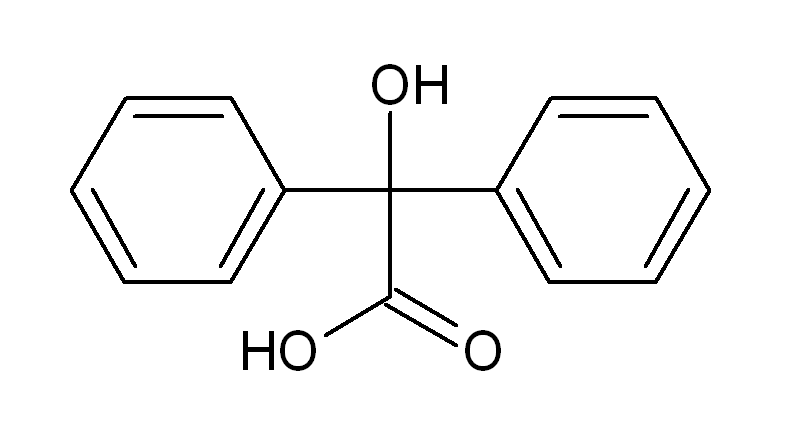
Benzilic acid is an aromatic carboxylic acid with the chemical formula C6H5CH(CO2H)2. It is a white crystalline solid that is soluble in water and organic solvents. Benzilic acid is an intermediate in the synthesis of a variety of chemicals and pharmaceuticals, and it is also used as a food and flavor additive.
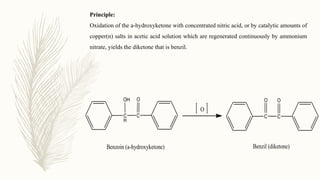
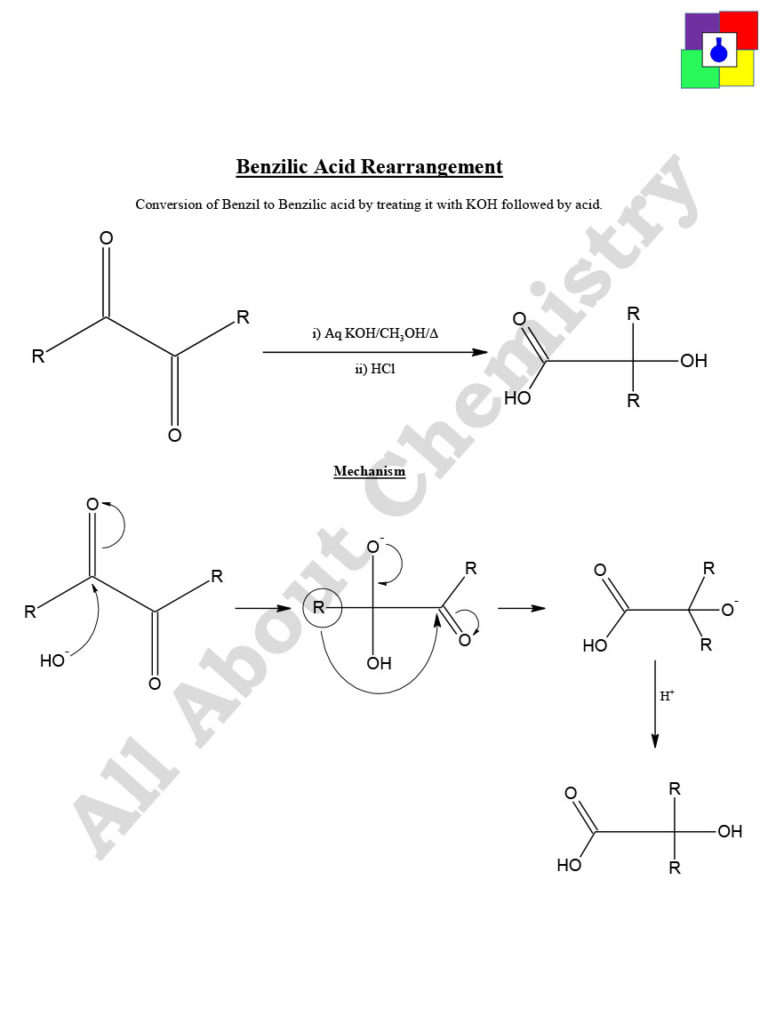
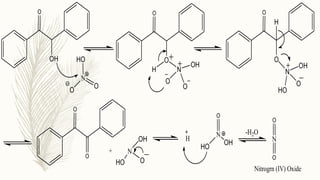
There are several methods for preparing benzilic acid, each with its own advantages and disadvantages. One common method involves the base-catalyzed dehydration of benzil, which is an organic compound with the formula C6H5CO2C6H5. Benzil can be synthesized from benzaldehyde, which is an aromatic aldehyde with the formula C6H5CHO, through a process known as the benzoin condensation.
To prepare benzilic acid from benzil, the first step is to dissolve the benzil in a solvent such as ethanol or methanol. A strong base, such as sodium hydroxide or potassium hydroxide, is then added to the solution to catalyze the dehydration reaction. The base promotes the elimination of water from the benzil molecule, resulting in the formation of benzilic acid and the corresponding alcohol.
The yield of benzilic acid can be improved by adding a small amount of a dehydrating agent, such as sulfuric acid or phosphoric acid, to the reaction mixture. The dehydrating agent helps to remove the water by-product from the reaction, which can otherwise inhibit the formation of benzilic acid.
After the dehydration reaction is complete, the benzilic acid can be separated from the solvent and other reaction by-products by filtration or crystallization. The purity of the benzilic acid can be determined by various analytical techniques, such as spectroscopy or chromatography, to ensure that it meets the desired specifications.
In conclusion, benzilic acid is an important chemical intermediate that can be prepared through the base-catalyzed dehydration of benzil. The yield and purity of the final product can be optimized through the use of appropriate solvents, bases, and dehydrating agents, as well as by carefully controlling the reaction conditions.
IL34962A
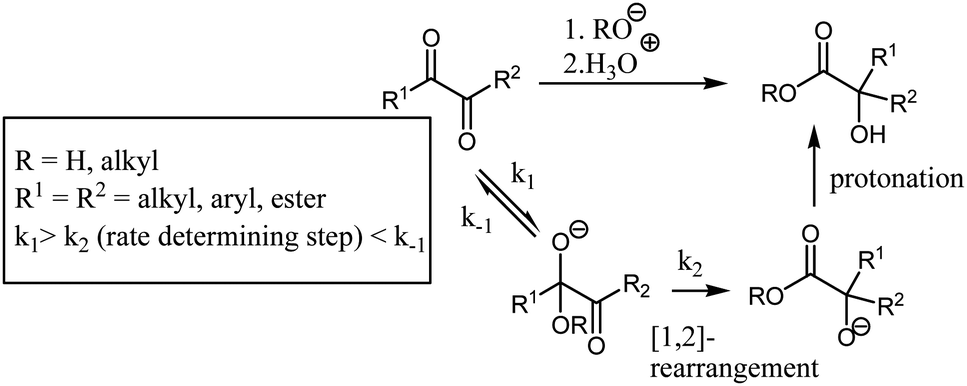
From above table data, the preparation method of benzilic acid of the present invention has saved point this step of oily matter in existing preparation method, but expects to obtain benzilic acid crude product by directly getting rid of after hydrochloric acid acid adjustment, has simplified operation, provides cost savings; And centrifugal drying material obtains benzilic acid finished product after benzilic acid crude product and alcohol reaction, and the purity of this benzilic acid finished product is high, and transmittance is good and productive rate is high. Where in the benzilic acid rearrangement carried out using potassium hydroxide in a mixture of ethanol-ether. Google has not performed a legal analysis and makes no representation as to the accuracy of the date listed. The pH of the solution is maintained between 6. Within 30 minutes, 116 g. It is aromatic because it has a benzene ring in its chemical structure.
Synthesis of benzilic acid from benzoin

However, the solvent free media has advantage that it reduces the volume of reaction mass. By the above table data as can be known, the preparation method of benzilic acid of the present invention has saved minute this step of oily matter among the existing preparation method, but expects to get the benzilic acid crude product by directly getting rid of after the hydrochloric acid acid adjustment, has simplified operation, provides cost savings; And the centrifugal drying material obtains the benzilic acid finished product behind benzilic acid crude product and the alcohol reaction, and the purity of this benzilic acid finished product is high, and transmittance is good and productive rate is high. The other metal hydroxides have also been reported for benzilic acid rearrangement. A total of 750—800 cc. Another disadvantage is that this method requires rectified spirit.
Preparation of Benzilic acid
Benzoic acid is an organic compound because it contains carbon and it is also an aromatic carboxylic acid. The temperature ranging from 20° C. However, using this procedure, diflieult waste water problems were encountered. As heating continues the mixture thickens and more water is added from time to time. The beneficial effect that the technical scheme that the embodiment of the present invention provides is brought is: the preparation method of benzilic acid of the present invention has saved point this step of oily matter in existing preparation method, but expect to obtain benzilic acid crude product by directly getting rid of after hydrochloric acid acid adjustment, simplify operation, provided cost savings; And centrifugal drying material obtains benzilic acid finished product after benzilic acid crude product and alcohol reaction, and the purity of this benzilic acid finished product is high, and productive rate is high. Turn in the entire sample to the instructor in a labeled vial. However, your sample should be dry enough to proceed to the next experiment making benzil.
CN102924264A

It is a solid that is crystalline in appearance, similar to white needles. The yield of the product is comparable in both the media. You must use a mL round bottom flask for this oxidation reaction. It should be pointed out that for the person of ordinary skill of the art, without departing from the inventive concept of the premise, can also make some distortion and improvement, these all belong to protection scope of the present invention. The final benzilic acid products thus obtained such as, for example, 4,4'-dibromobenzilic acid, 4,4-dichlorobenzilic acid, and the like, are then esterified by conventional procedures that is, by reaction with an ester of a low aliphatic alcohol or an aromatic alcohol in the presence of an inert solvent. But the yield that use aforesaid method is prepared benzilic acid is low, and the purity of the benzilic acid of gained is not high yet.