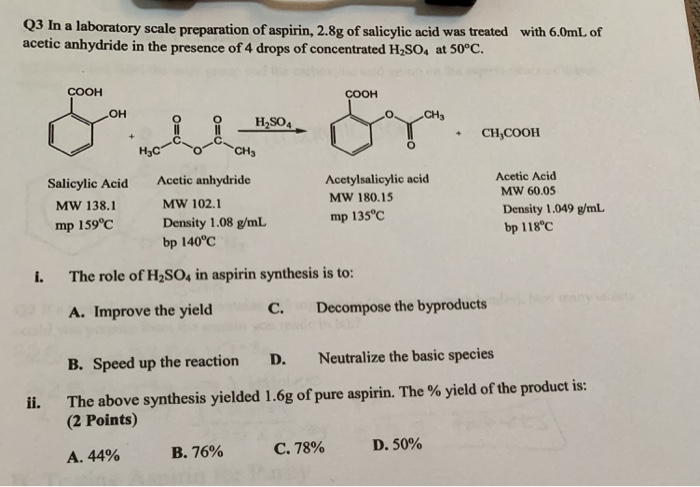
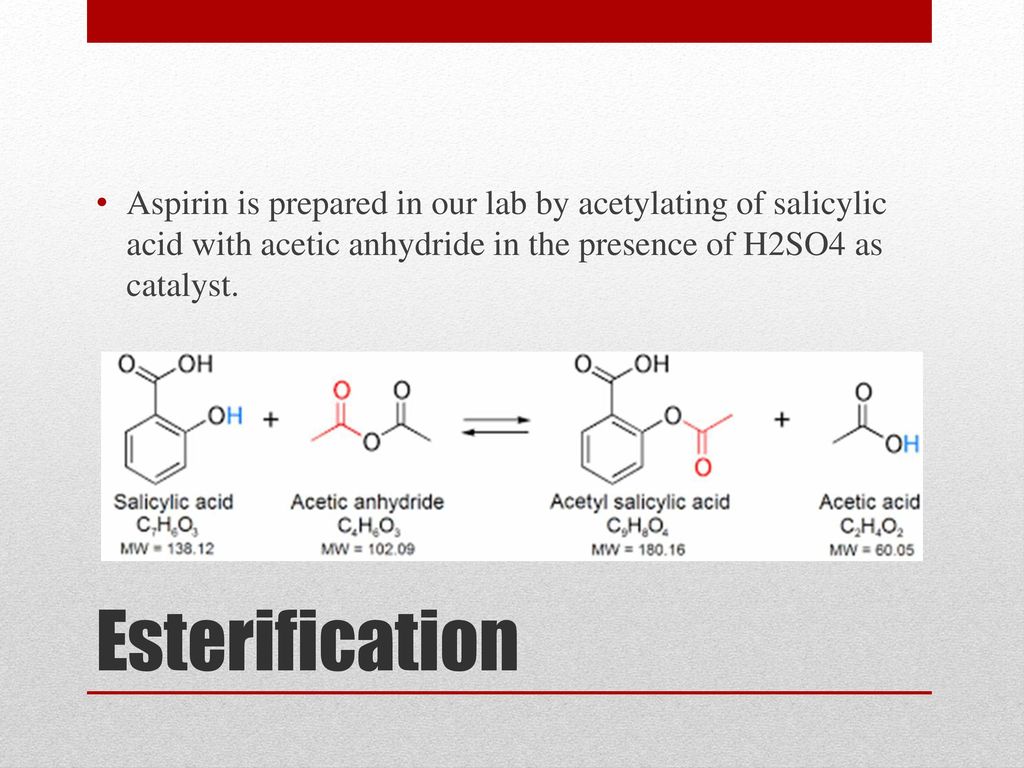
Aspirin is a commonly used pain reliever and anti-inflammatory medication. It is also known as acetylsalicylic acid, and is derived from salicylic acid, a compound found in plants such as willow trees. The preparation of aspirin involves a chemical reaction between salicylic acid and acetic anhydride, with the addition of a small amount of sulfuric acid as a catalyst.
To prepare aspirin, the following materials and equipment are needed:
- Salicylic acid
- Acetic anhydride
- Sulfuric acid
- Beaker
- Stirring rod
- Flask
- Filter paper
- Glass funnel
The first step in preparing aspirin is to measure out the appropriate amounts of salicylic acid and acetic anhydride. These two compounds are mixed together in a beaker, along with a small amount of sulfuric acid as a catalyst. The mixture is then stirred thoroughly with a stirring rod to ensure that the compounds are well combined.
Next, the mixture is transferred to a flask, and heated over a Bunsen burner until the reaction is complete. The temperature of the mixture should be kept at around 70-80°C, and the reaction should be allowed to continue for about an hour.
After the reaction is complete, the mixture is allowed to cool and then filtered through a piece of filter paper. The resulting solid product is aspirin, which can be collected by pressing it out of the filter paper using a glass funnel.
It is important to note that the preparation of aspirin requires careful handling of chemicals, as some of the compounds used are potentially dangerous. Proper protective equipment, such as goggles and gloves, should be worn at all times during the preparation process.
Overall, the preparation of aspirin from salicylic acid is a simple chemical reaction that can be easily carried out in a laboratory setting. The resulting product, acetylsalicylic acid, is a widely used pain reliever and anti-inflammatory medication that is effective in reducing the symptoms of various conditions such as headache, fever, and inflammation.