The potato osmosis lab is a classic science experiment that demonstrates the movement of water through a semi-permeable membrane. This experiment is often used to teach students about the concept of osmosis, which is the movement of water from a high concentration to a low concentration through a semi-permeable membrane. It can also be used to teach students about the properties of cell membranes and the role they play in the transport of substances in and out of cells.
To perform the potato osmosis lab, you will need the following materials: potatoes, water, salt, sugar, beakers, graduated cylinders, and a balance scale. You will also need a sharp knife to cut the potatoes into thin slices.
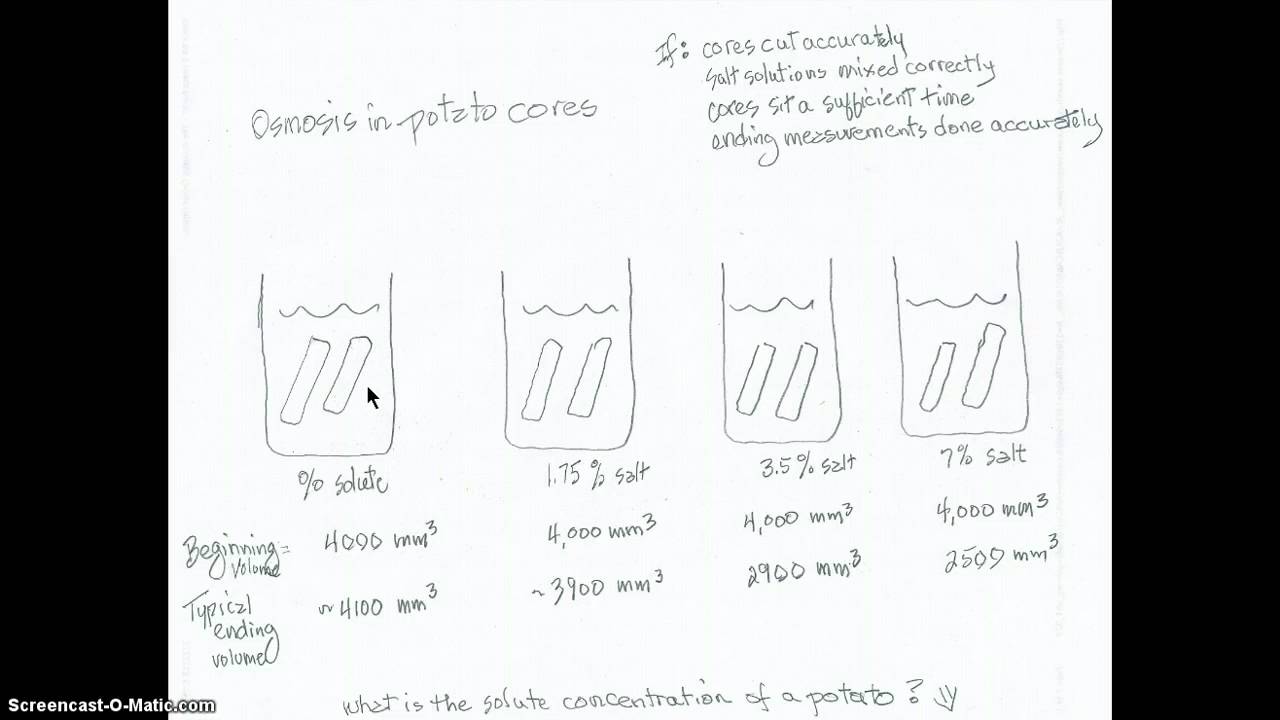
To begin the experiment, you will need to cut the potatoes into thin slices and weigh them on the balance scale. Record the weight of each potato slice. Next, you will need to prepare four different solutions by mixing water with different concentrations of salt and sugar. One solution should be pure water, one should contain a high concentration of salt, one should contain a high concentration of sugar, and one should contain a combination of both salt and sugar.
Once the solutions are prepared, you will need to place the potato slices into the different solutions and allow them to sit for a certain amount of time. After the allotted time has passed, you will need to remove the potato slices from the solutions and weigh them again.
As the potato slices sit in the different solutions, water will move in or out of the cells through the semi-permeable membrane. If the potato slice is placed in a solution with a higher concentration of water, water will move into the cells through osmosis. If the potato slice is placed in a solution with a lower concentration of water, water will move out of the cells through osmosis.
You can use the data collected from the experiment to create a graph that shows the movement of water in and out of the potato slices. This will allow you to see how the concentration of the solution affects the movement of water through the semi-permeable membrane.
The potato osmosis lab is a simple and effective way to teach students about the concept of osmosis and the role of cell membranes in the transport of substances. It is a hands-on experiment that allows students to see the movement of water in and out of cells and to understand how different concentrations of substances can affect this movement.