Niccolò Machiavelli was a Renaissance political philosopher and statesman whose ideas continue to influence political thought to this day. One of the key concepts in his philosophy is the idea of fortune, or Fortuna in Italian. This concept plays a central role in his most famous work, The Prince, in which he advises rulers on how to acquire and maintain power.
According to Machiavelli, Fortuna is a fickle and unpredictable force that can either help or hinder a ruler's efforts to achieve their goals. He believed that Fortuna was beyond human control and could not be relied upon to bring success. Instead, he argued that a ruler should focus on their own actions and abilities, and not rely on Fortuna to deliver them victory.
Machiavelli argued that Fortuna could be harnessed to a certain extent through the use of virtù, or personal ability and courage. A ruler with virtù could take advantage of opportunities presented by Fortuna and use them to further their own ends. However, he also recognized that Fortuna could be a double-edged sword, and that a ruler who relied too heavily on it could be led astray and ultimately fail.
In The Prince, Machiavelli advises rulers to be cautious in their dealings with Fortuna, and to be prepared for both success and failure. He advises them to have contingency plans in place in case things do not go as expected, and to be flexible and adaptable in the face of changing circumstances.
Overall, Machiavelli's concept of Fortuna is a reminder that success is not always within our control, and that we must be prepared to deal with both good and bad luck as it comes our way. It is a cautionary tale for those who seek power and influence, and a reminder of the importance of personal responsibility and agency in achieving our goals.
Potato Osmosis Lab Report Example (500 Words)
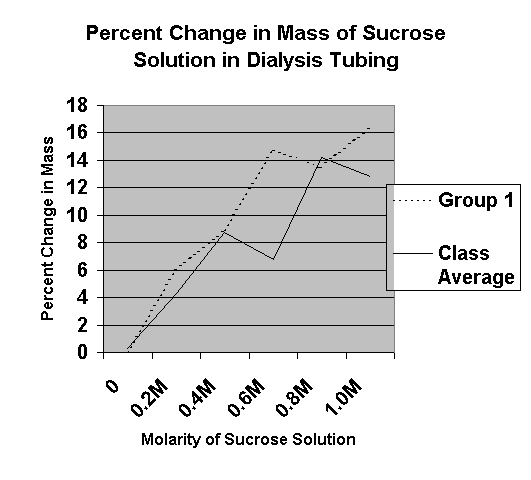
The next day, the potato pieces are removed from the solutions, blotted dry, and their final masses are recorded. How do you know? Students will observe cells under the microscope as plasmolysis takes place, and easily and excitedly view the collapse of the cell. Questions: What causes sugar to shrink and swell things? If the solute concentration is high, then the water concentration will be low by comparison. As an improvement, if we were able to have more time then we would have been able to produce more results and data points which could further improve the analysis providing stronger evidence. INTRODUCTION The cell membrane surrounds the cell and is responsible for the regulation of substances within the cell.
The Osmosis Lab : 14 Steps

The distilled water was hypotonic compared to the potato cells that contain approximately 2% salt. In which osmosis occurs to balance between the concentration of water in a substance and in the air which adds water to air and this makes the air denser. This would explain the physical changes — the increase in mass and length as well as the increase in turgidity - in the potato strips immersed in 100% H2O solutions or low NaCl-concentration solutions. A hypotonic solution is one with a lower osmotic pressure, indicating that the net movement of water moves into the said solution whereas a hypertonic solution is one with a higher osmotic pressure, thus the net movement of water will be leaving the hypertonic solution. Osmosis only deals with water and is a type of diffusion. Moreover, the potato cores mass will increase due to adding more amounts of sucrose, because when adding more amounts of sucrose the potato will swallow more sucrose which has a certain mass that will be added to the potato.
Effect of Salt Concentration on Osmosis in Potato Cells Lab Answers

Nevertheless, there are several possible sources of error that could have greatly or negligibly affected the outcome of the experiment. Water boils to produce steam at 100 C 212 F b. Life cannot be imagined without the implication of technological innovation. To further better this experiment, I would extend the time over a course of 2 hours and would test the experiment outside on the sun. Therefore, as NaCl solution is less concentrated in H2O molecules than the potato strips, the decrease in mass and length and loss of turgidity results from the net movement of water leaving the potato strips, which is higher in osmotic pressure, and diffusing into the solution.
Sample Lab Report

The potatoes are allowed to sit in their various solutions overnight. The variables recorded for each potato piece are Lab Group Name, Sucrose Concentration Molarity , Initial Mass g , Final Mass g , and Mass Change %. Primarily, these would include varying temperatures and humidity which could potentially affect the rate of osmosis as increased temperature results in increased diffusion while increased humidity results in an increased number of water molecules. With the use of a potato, this experiment is an informative way to comprehend the overall effect of different concentrations of NaCl has on potatoes, while also determining the concentration of the solute in the pieces of potatoes. With the potato cores in the beaker we then put a watch glass over the top of the beaker to minimize the amount of solution that evaporates. Alternatively, the decrease in mass and length in the potato strips submerged in highly concentrated NaCl solutions can be explained by its immersion in a hypotonic solution.