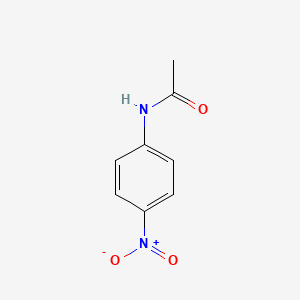
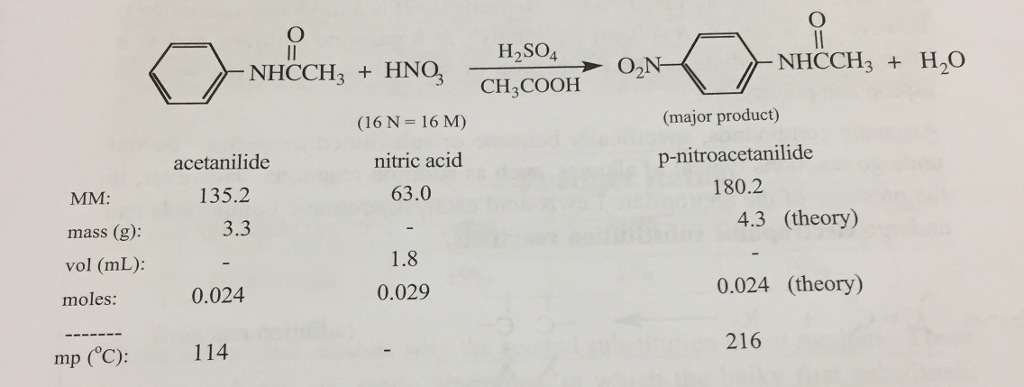Para-nitroacetanilide (p-nitroacetanilide) is an organic compound that is derived from acetanilide. It is a white, crystalline solid that has the formula C8H9NO3. The compound is typically synthesized through a nitration reaction, where acetanilide is treated with a mixture of concentrated nitric acid and sulfuric acid.
The nitration reaction results in the replacement of one of the hydrogen atoms on the acetanilide molecule with a nitro group (-NO2). This results in the formation of p-nitroacetanilide, as well as a small amount of m-nitroacetanilide and o-nitroacetanilide. The yields of these three compounds depend on the conditions of the nitration reaction, with p-nitroacetanilide typically being the most prevalent.
P-nitroacetanilide is a useful intermediate in the synthesis of a variety of compounds, including dyes, pigments, and pharmaceuticals. It can also be converted back to acetanilide through a reduction reaction, which involves the removal of the nitro group. This reaction can be carried out using reducing agents such as hydrazine or sodium borohydride.
P-nitroacetanilide is a relatively stable compound, but it can decompose if subjected to high temperatures or strong acids. It is also sensitive to light and should be stored in a cool, dry place to prevent degradation.
In conclusion, p-nitroacetanilide is a useful organic compound that is derived from acetanilide through a nitration reaction. It is used as an intermediate in the synthesis of a variety of compounds and can be converted back to acetanilide through a reduction reaction. Despite its stability, it should be stored and handled with care to prevent degradation.
To Prepare a Sample of p

Before moving further, here is a summary of acetanilide. After adding all the mixed acid, remove the beaker from the freezing mixture and keep it for 1 hr at room temperature. Add dry acetanilide 25 g to glacial acetic acid 25 ml in a beaker and then introduce concentrated sulphuric acid 50 ml slowly with constant stirring to obtain clear solution. Anyway, the product was dissolved, with great difficulty, using heating. Generally, it is slightly yellowish.
Preparation of p

So, we got to do a nitration ages ago in organic chemistry labs, which was a whole bunch of fun. HN0 3 since —NH 2 group gets oxidised which is not required. Nitroacetanilide is a combination of Nitro and Acetanilide. All aspects of p-Nitroacetanilide, including general information, preparation, and properties, are discussed in this curated p-Nitroacetanilide study material. Owing to this, the Para Nitroacetanilide gets easily crystallised.
Synthesis of p nitroacetanilide from acetanilide

Since o-nitro acetanilide is much soluble in alcohol, it is so easy to isolate p-nitro acetanilide through crystallization. Still though, we got what LOOKED to be a clean product out. Para nitro acetanilide is also referred to as 4-Nitroacetanilide. As a result, purified p-Nitroacetanilide is obtained, and the melting point is also determined. To Prepare a Sample of p-Nitro acetanilide from Acetanilide Theory The nitration of aniline is difficult to carry out with nitrating mixture a mixture of cone. However, excessive consumption or exposure can lead to respiratory and skin problems. Hence, it is named Para Nitroacetanilide.
Synthesis of p
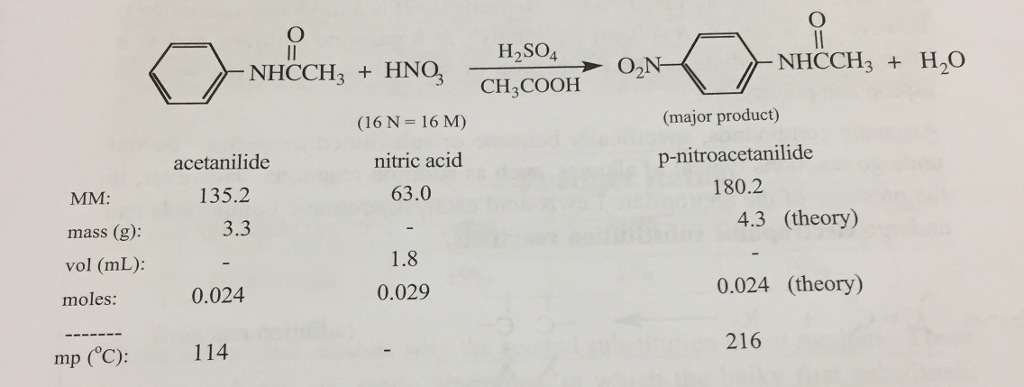
During crystallisation, the product obtained from nitration contains two types of acetanilide, namely ortho and para. It can be used to manufacture dyes, medicines, anti-oxidants, and gasoline, which have become essential in current times. Filter while hot and cool the filtrate in ice. In the second step p-nitroaniline is prepared from p-nitro acetanilide due to hydrolysis of acetate ion from acetamido functional group in presence of concentrated sulphuric acid. Conclusion As we came so far, we learned all the aspects of p-Nitroacetanilide. After this, a purification process is processed to obtain pure P-Nitroacetanilide.
p

An organic substance, acetanilide, is processed through nitration to prepare p-Nitroacetanilide. Moreover, the compound is often known as 4-Nitroacetanilide, P-Acetamido Nitrobenzene, N- 4-nitrophenyl acetamide and N-Acetyl-4-nitroaniline. Dissolve the crude product obtained above in about 20 ml of methylated spirit. Deuterochloroform CDCl 3 is defined as toxic, so it also should be handled with care. Introduction to Preparation of p-Nitroacetanilide Nitration is an important reaction that is used within the training of nitro compounds.
HCl is defined as corrosive; hence, avoid all contact and handle this compound with caution. What is the Colour of the product in the preparation of P Nitro Acetanilide from acetanilide? So, o-nitroacetanilide remains in the initial solvent and during crystallization only p-nitroacetanilide forms crystals and separates out from the mixture. Stir the contents and wait until the temperature becomes less than 5°C. Moreover, the glacial acetic acid is also used during preparation, as it possesses the properties of a polar solvent, which regulates the acetanilide dissolving. Filter while hot and cool the filtrate in ice. Conclusions: Never trust a lab partner, always double check things. Nitro compounds are used as beginning substances for lots of commercially beneficial materials consisting of dyes, pills, explosives, and so forth.