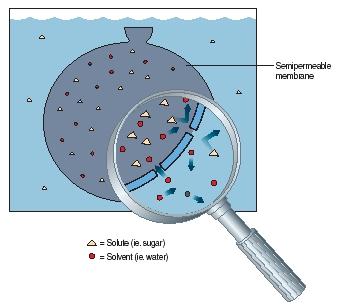
Osmosis is the process by which water molecules move across a semipermeable membrane from an area of high water concentration to an area of low water concentration. This process is essential for the survival of many living organisms, as it plays a crucial role in regulating the balance of water and other substances within the body.
In humans and other animals, osmosis helps to maintain proper hydration and electrolyte balance. Water moves into and out of cells through the cell membrane, which acts as a selective barrier that allows certain substances to pass through while preventing others from entering or leaving. The movement of water across the cell membrane is determined by the concentration gradient, which refers to the difference in concentration of a particular substance on either side of the membrane. When the concentration of a substance is higher on one side of the membrane, water will tend to move towards the side with the lower concentration in an attempt to equalize the concentration of the substance on both sides.
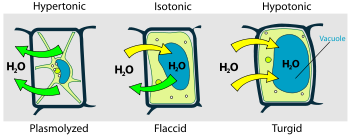
One important function of osmosis in living organisms is the regulation of cell volume. When cells are placed in a solution with a higher concentration of solutes, they will tend to lose water through osmosis, causing them to shrink. Conversely, when cells are placed in a solution with a lower concentration of solutes, they will tend to gain water through osmosis, causing them to swell. This process is essential for maintaining the proper shape and function of cells, as well as for regulating the overall hydration of the body.
Osmosis also plays a role in the transport of substances across cell membranes. Many substances, such as glucose, are too large to pass through the cell membrane by themselves. Instead, they must be transported across the membrane using special proteins called transporters. These proteins bind to the substance and transport it across the membrane, a process that requires the movement of water molecules. In this way, osmosis helps to facilitate the movement of essential nutrients and waste products in and out of cells.
In addition to its role in regulating hydration and the transport of substances, osmosis is also important for the function of organs and tissues in the body. For example, osmosis plays a key role in the filtration of blood in the kidneys, which helps to maintain the proper balance of water and electrolytes in the body. It is also involved in the absorption of water and nutrients in the digestive tract and the regulation of blood pressure in the circulatory system.
In summary, osmosis is a vital process that plays a crucial role in the function of many living organisms. It helps to regulate the balance of water and other substances within the body, maintain the proper shape and function of cells, and facilitate the transport of essential nutrients and waste products. Without osmosis, many of the essential processes that keep living organisms healthy and functioning would not be possible.