Nylon is a synthetic polymer that was first developed in the 1930s by Wallace Carothers, a scientist working at DuPont. It is a versatile material that has a wide range of applications, including in clothing, automotive parts, and medical devices.
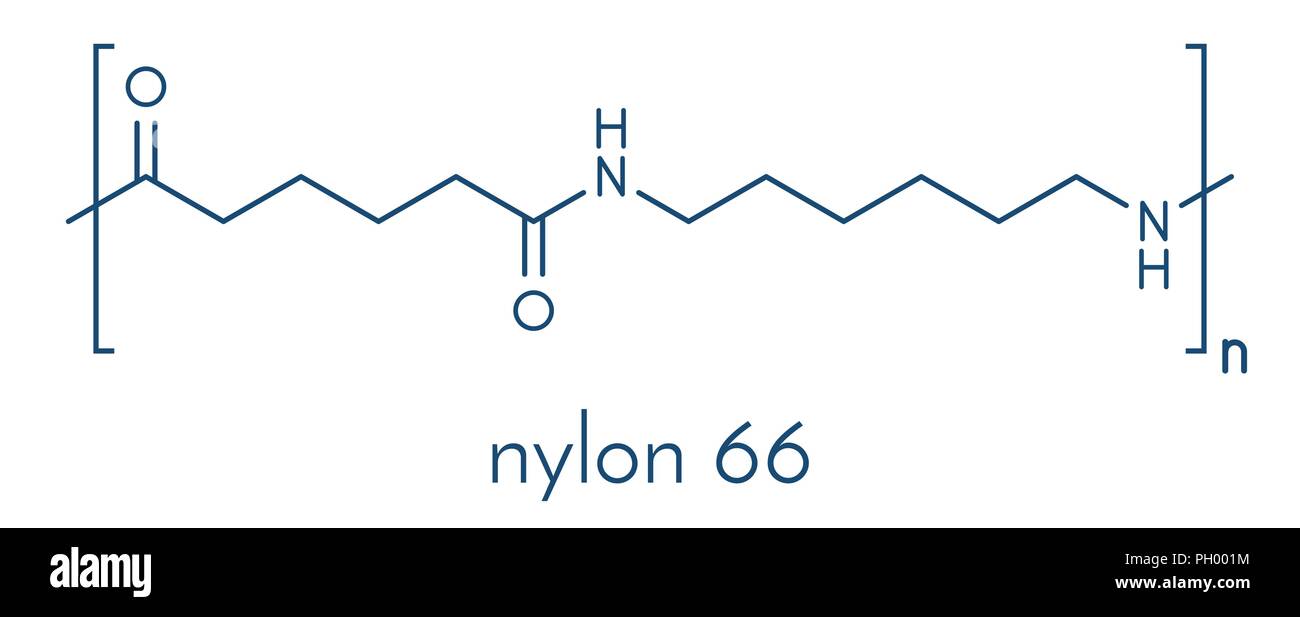
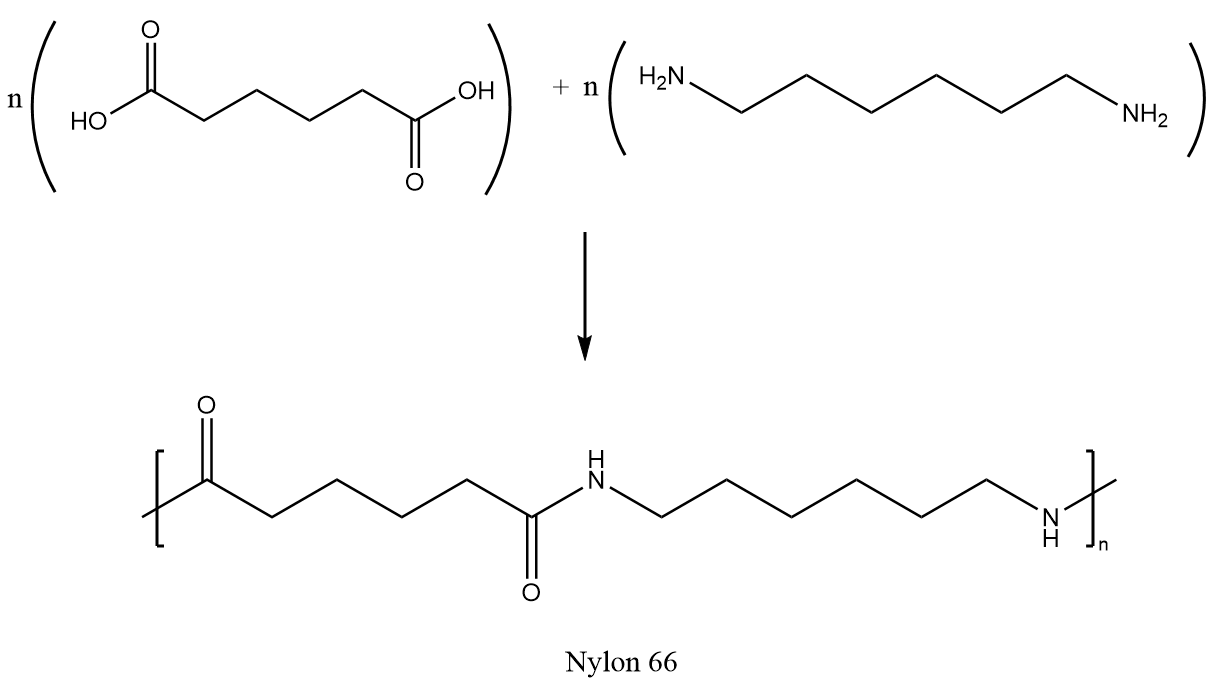
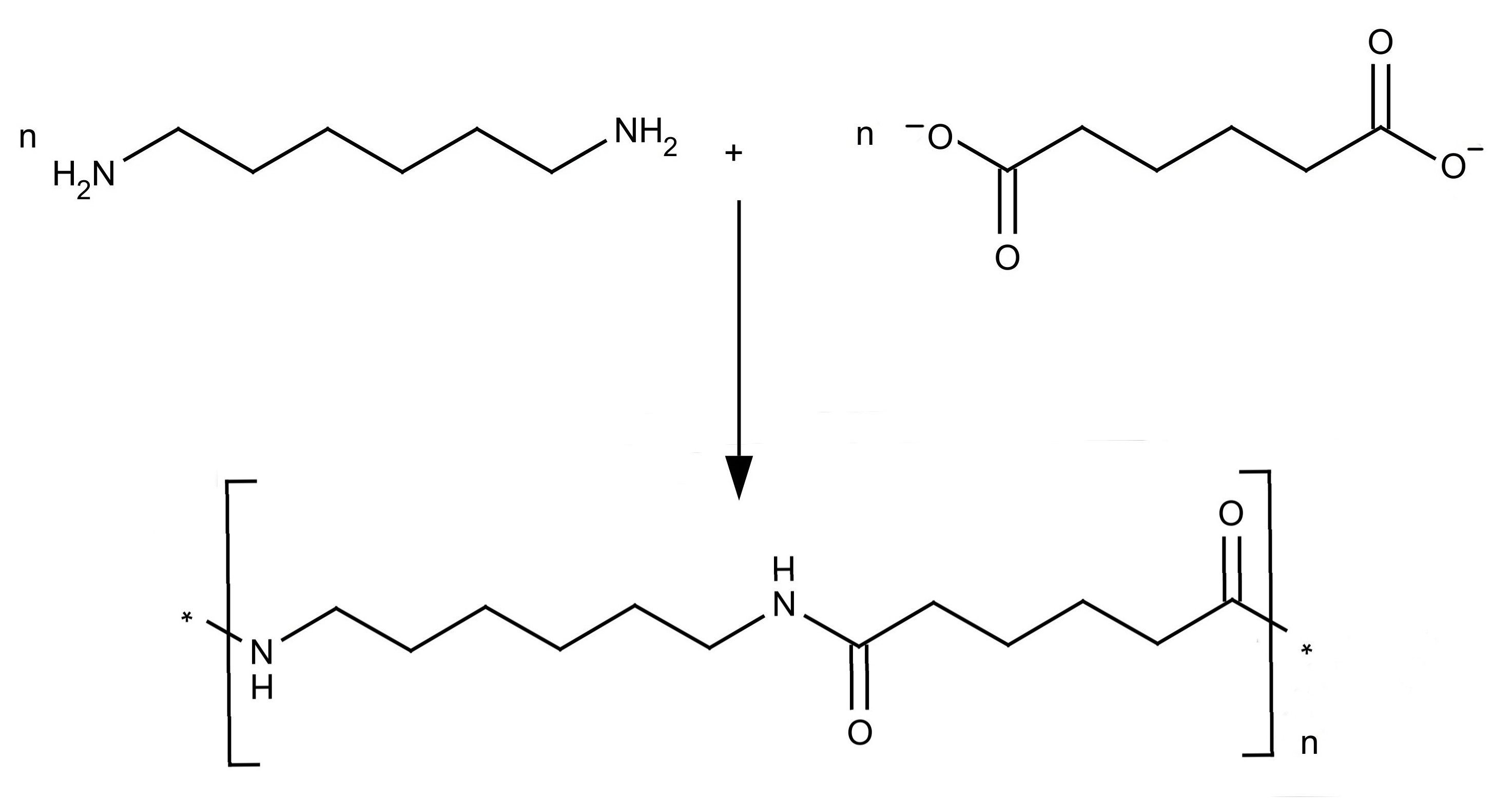
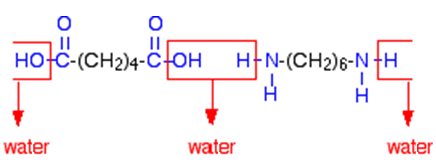
Nylon is made through a process called polymerization, which involves the chemical reaction between monomers to form a polymer chain. The monomers used to make nylon are amides, which are compounds that contain a nitrogen atom bonded to a carbon atom. These monomers are joined together through a process called condensation polymerization, in which water is released as a byproduct.
There are several different types of nylon, each of which has its own unique properties and applications. For example, nylon 6,6 is a type of nylon that is made from two different monomers, hexamethylenediamine and adipic acid. It is known for its high strength and durability, making it ideal for use in clothing, automotive parts, and other applications that require these properties.
Nylon can be molded into a variety of shapes and forms, including fibers, films, and sheets. It is also resistant to abrasion and has good chemical resistance, making it a popular choice for use in a variety of industrial and consumer products.
In addition to its many practical uses, nylon also has environmental benefits. It can be recycled and reused, and it is biodegradable, meaning it can break down naturally in the environment.
Overall, nylon is a valuable and versatile material that has a wide range of applications. Its strength, durability, and versatility make it an important material in a variety of industries, and its environmental benefits make it a sustainable choice for many applications.