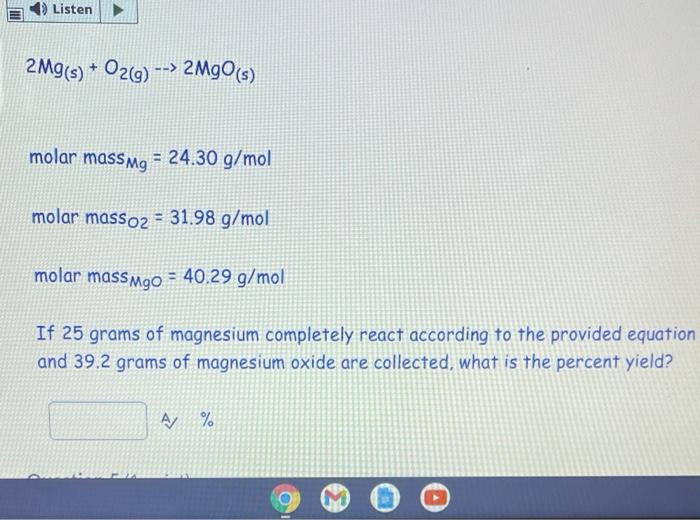
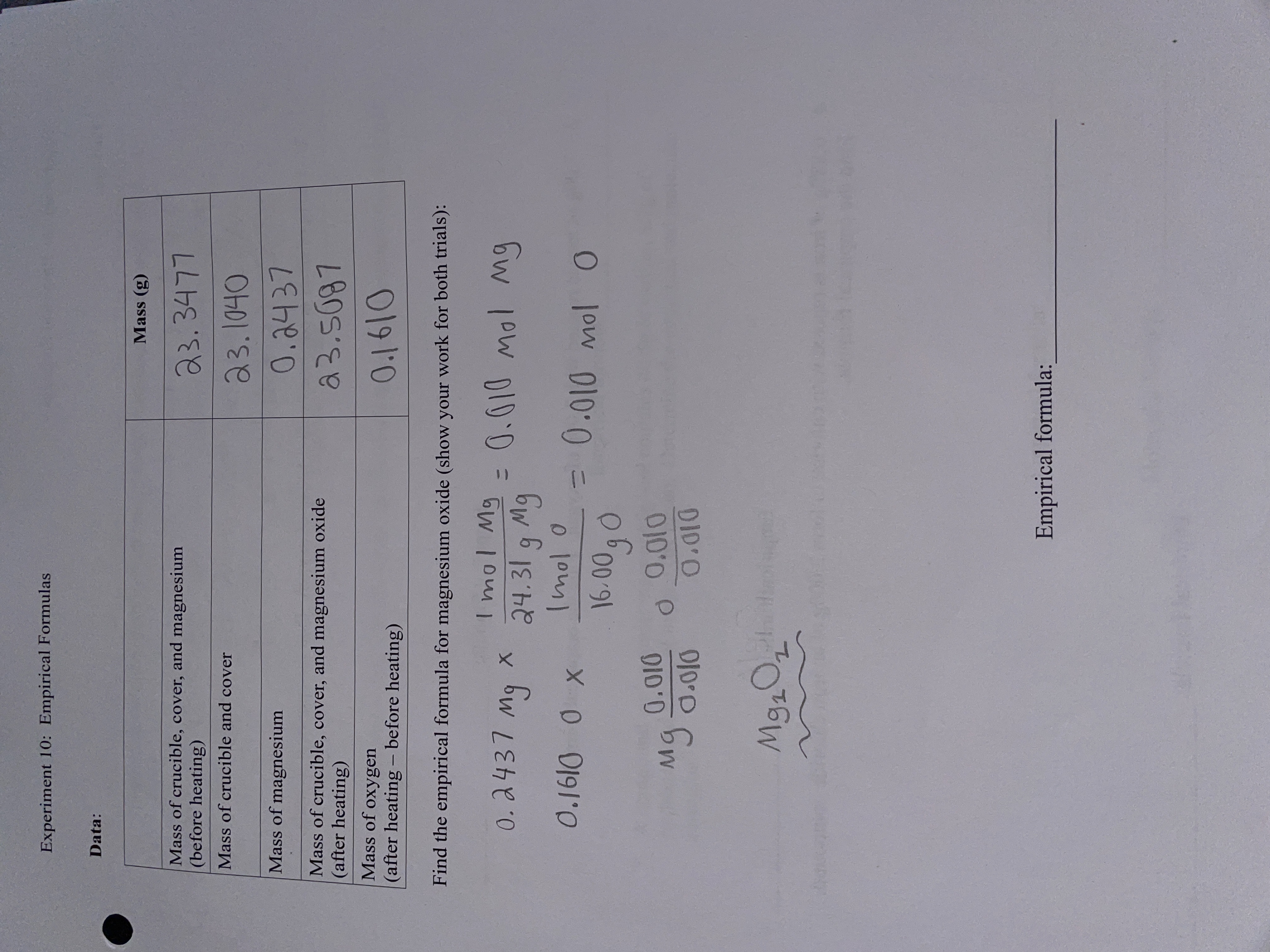
Molar mass is a measure of the amount of matter contained in a substance, and it is typically measured in grams per mole (g/mol). The molar mass of a substance can be determined by summing the atomic masses of all the atoms present in the substance. In the case of magnesium oxide (MgO), the molar mass can be calculated by adding the atomic mass of magnesium (24.3050 g/mol) to the atomic mass of oxygen (15.999 g/mol). The resulting value is the molar mass of MgO, which is 40.3050 g/mol.
Magnesium oxide is a chemical compound that is formed when magnesium reacts with oxygen. It is a white, solid compound that is often used in the production of ceramics, refractories, and other materials. MgO is also used as a dietary supplement, as it is believed to have a number of health benefits.
One of the key characteristics of MgO is its high melting point, which is approximately 2800°C. This makes it useful in a number of high-temperature applications, such as in the production of steel and aluminum. MgO is also highly resistant to chemical attack, making it useful as a protective coating for a variety of materials.
In addition to its industrial uses, MgO has a number of medical applications. It is commonly used as an antacid to neutralize excess stomach acid, and it is also used as a laxative to relieve constipation. MgO is also believed to have anti-inflammatory properties, and it is sometimes used to treat conditions such as asthma and eczema.
Overall, the molar mass of MgO is an important characteristic of this chemical compound. It allows scientists and engineers to accurately measure and control the amount of MgO present in a given sample, which is critical in a variety of applications.