There are several methods that can be used to determine the concentration of iron in unknown samples. These methods are based on different chemical and physical properties of iron and can be used to determine the amount of iron present in various types of samples, including biological, environmental, and industrial samples.
One common method for determining the concentration of iron in a sample is through the use of atomic absorption spectroscopy (AAS). This technique involves the use of a spectrometer to measure the absorption of light by the atoms of a particular element in a sample. When a sample is placed in the spectrometer and exposed to a specific wavelength of light, the atoms of the element will absorb a certain amount of the light. The amount of light absorbed is proportional to the concentration of the element in the sample.
Another method for determining the concentration of iron in a sample is through the use of inductively coupled plasma mass spectrometry (ICP-MS). This technique involves the use of an inductively coupled plasma, which is a high-temperature gas plasma that is generated by an electrical current. The sample is introduced into the plasma, where the atoms are ionized and separated based on their mass-to-charge ratio. The ions are then detected by a mass spectrometer, which allows the concentration of the element to be determined.
A third method for determining the concentration of iron in a sample is through the use of colorimetry. This technique involves the use of a chemical reaction to produce a colored compound, the intensity of which is proportional to the concentration of the element in the sample. One common colorimetric method for determining the concentration of iron in a sample is through the use of the ferrozine assay, which involves the reaction of ferrozine with iron to produce a purple-colored compound.
There are also several other methods that can be used to determine the concentration of iron in a sample, including spectrophotometry, X-ray fluorescence, and neutron activation analysis. Each of these methods has its own specific advantages and limitations, and the choice of method will depend on the type of sample being analyzed and the concentration of iron present in the sample.
In conclusion, there are several methods that can be used to determine the concentration of iron in unknown samples, including AAS, ICP-MS, colorimetry, spectrophotometry, X-ray fluorescence, and neutron activation analysis. The choice of method will depend on the specific needs of the analysis and the characteristics of the sample being analyzed.
[PDF] Determination of Iron Species in Samples of Iron

We will use potassium permanganate, KMnO4, as the titrant in the analysis of an unknown sample containing iron to determine the percent iron by mass in the sample. Creating the optical signal involving the use of a transmitter, relaying the signal along the fiber, ensuring that the signal does not become too distorted or weak, receiving the optical signal, and converting it into an electrical signal. . It can build up in your dishwasher and discolour ceramic dishes. Ocean Optics Spectrophotometric Block Diagram Source: Is a tungsten-halogen lamp, which emits radiation in the visible region as well as the near the infrared and long wavelength end of the UV. An online tutorial of this lab is available Procedure Note: do this lab with a partner. These were first available in 2000 and with this technology the first Fibre-Optic Telecommunications System was developed.

Sample Holder: the sample holder that is also called the cuvette is a rectangular tube with two transparent faces. Slit: the entrance slit determines the spatial width of the light beam striking and grating. . In order to determine the Fe II concentration, a series of solutions of known concentrations were made. Expand It is found that ferritin is a slow release source of iron, readily available to humans or animals, based on RBC iron incorporation, and recognition of a second, nonheme iron absorption process, Ferritin endocytosis, emphasizes the need for more mechanistic studies onFerritin iron absorption and highlights the potential of ferritIn present in foods such as legumes to contribute to solutions for global iron deficiency.
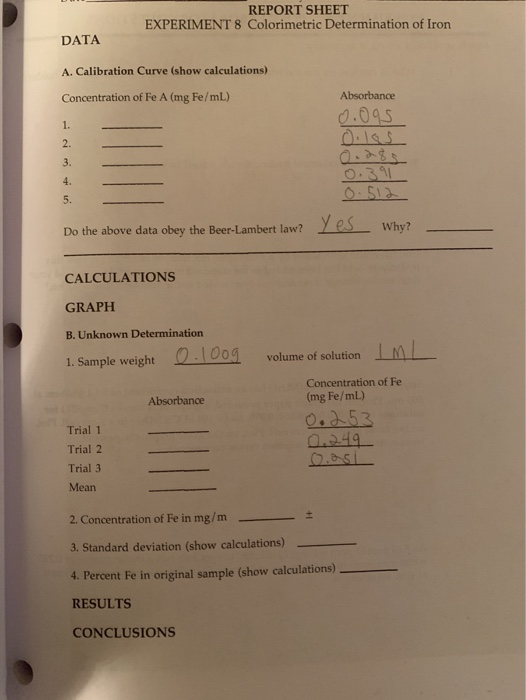
The percent error is the absolute difference between the experimental and theoretical value divided by the theoretical value, then multiplied by 100 to get a percent. It is not possible to prepare a KMnO4 standard solution based on a mass: Solid potassium permanganate cannot be obtained in a completely pure state due to the high reactivity mentioned above. Sample Unknown Preparation The sample preparation requires crushing a pill, boiling the powder in acid to release the iron, and filtering the solution to remove particulates that could interfere in the spectrophotometry measurements. Although you should use this time wisely, I advise against cleaning up, as you may need to remake a solution or two before the lab ends. The procedure of sample extraction was developed and optimized, preserving the content of particular forms of iron. Thather,potassium permanganate solutions are prepared to be an approximate concentration, and are then standardized against a known primary standard sample of the same substance which is to be analyzed in the unknown sample. Note: you are using a concentrated phenanthroline solution that ensures it is in GREAT excess.