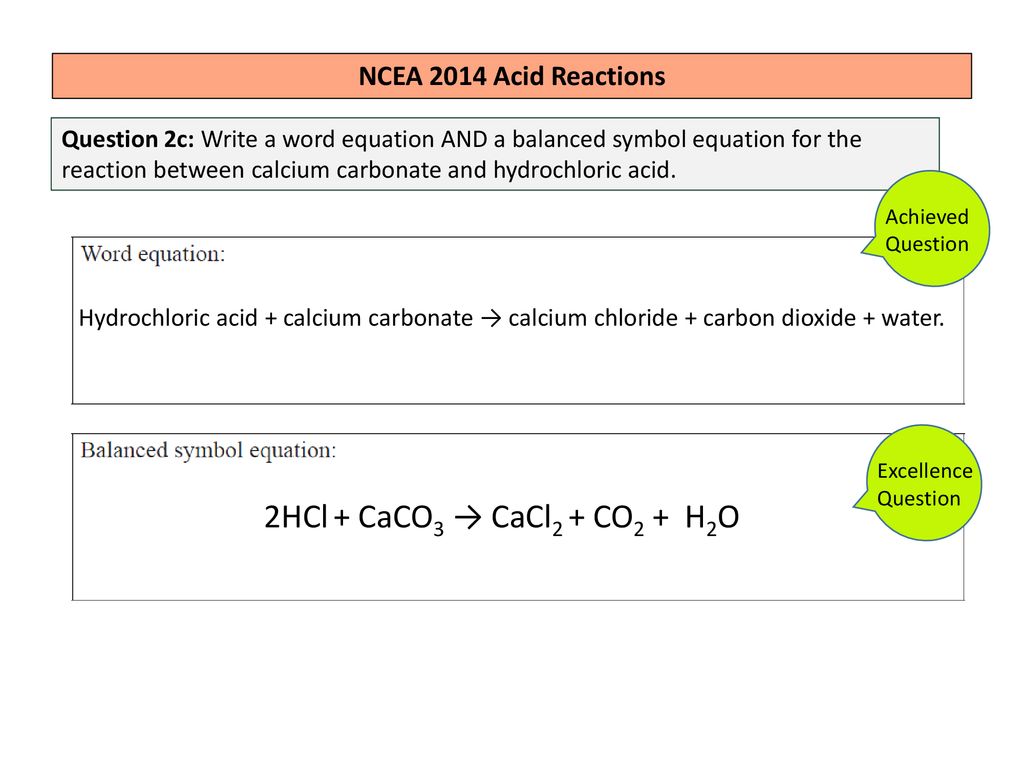
Marble chips are a type of rock that is composed primarily of calcium carbonate, a chemical compound with the formula CaCO3. When marble chips are placed in hydrochloric acid, a chemical reaction occurs that results in the production of several different products. The word equation for this reaction is:
Marble chips + hydrochloric acid -> calcium chloride + carbon dioxide + water
In this equation, the marble chips are represented by the symbol "Marble chips," and the hydrochloric acid is represented by the symbol "hydrochloric acid." The products of the reaction, which include calcium chloride, carbon dioxide, and water, are represented by the symbols "calcium chloride," "carbon dioxide," and "water," respectively.
The reaction between marble chips and hydrochloric acid is an example of a single displacement reaction, in which one element is replaced by another element. In this case, the calcium in the marble chips is replaced by the hydrogen in the hydrochloric acid, resulting in the production of calcium chloride. The carbon dioxide and water are also produced as byproducts of the reaction.
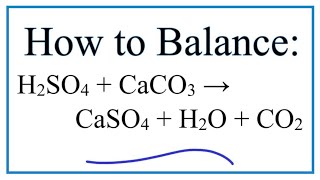
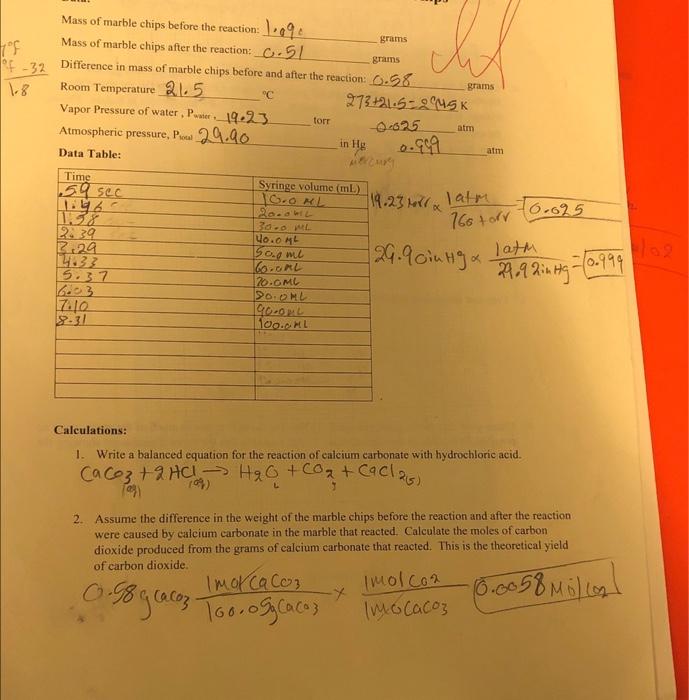
The reaction between marble chips and hydrochloric acid can be represented by the following balanced chemical equation:
CaCO3 + 2HCl -> CaCl2 + CO2 + H2O
In this equation, the symbols "CaCO3," "HCl," "CaCl2," "CO2," and "H2O" represent the reactants and products of the reaction, respectively. The coefficients in front of each compound indicate the relative number of atoms or molecules involved in the reaction.
Overall, the reaction between marble chips and hydrochloric acid is a simple but powerful example of how chemical reactions can produce new and useful products from simple starting materials. Understanding the principles behind this reaction can help us to better understand and predict the behavior of other chemical reactions, and to design new and more efficient processes for the production of chemicals and other materials.
What is the chemical reaction for marble chips with hydrochloric acid?

The mineral reacts with hydrochloric acid to produce carbon dioxide gas, water,… What happens when magnesite reacts with hydrochloric acid? If the reaction goes very quickly, I will take the readings every five seconds. As there is no clearly linear region in this plot, you can assume that at no time a second-order reaction occurs in the experiment. I will also make sure i keep the tops of the acid bottoms firmly on and keep them in the tray and away from the side of the table per venting acid spilling all over the floor. The Premium Chemical reaction Chemistry Chlorine. Investigation Planning Method First of all I will need to get out the following pieces of equipment, a gas syringe, a chronicle flask, a burette, a clamp, a 10 cm cubed measuring cylinder, a set of scales, a beaker, a stop watch, dilute hydrochloric acid and medium I will weigh out exactly or as close as possible to 1.
What happens if you put marble in hydrochloric acid?

If the hydrochloric acid touches your skin rinse with water straight away. As the chemical reaction occurred, the water in the measuring cylinder was displaced and gas bubbles that were blowing out represented carbon dioxide. As a result, the rate law must be determined experimentally. In order to determine the rate coefficient k, click the diagram with the right mouse button, select In the experiment example, the plot for the first-order rate equation does not result in a straight line over the whole range because, after part of the hydrochloric acid has reacted, the rate is governed by diffusion: when the concentration of hydrochloric acid decreases, the acid reacts at a faster rate than new H 3O + ions are transported to the surface of the marble. Marble is a carbonate rock, and therefore is soft less than your knife and fizzes. If there is still some magnesium left over when it has stopped effervescing then I will have to increase the volume of hydrochloric acid.