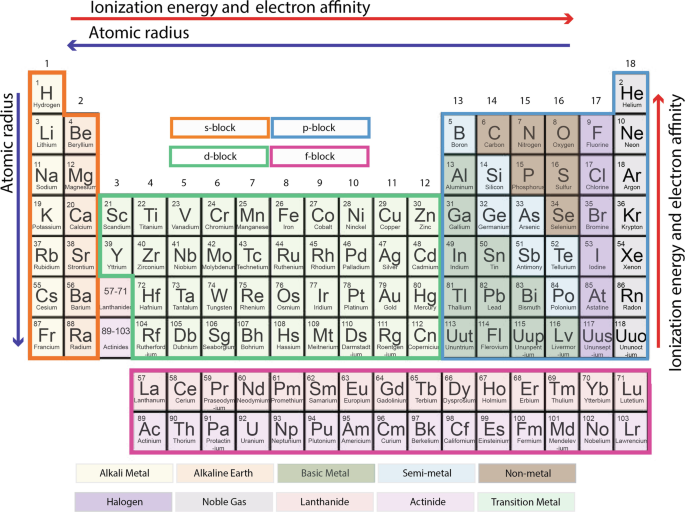
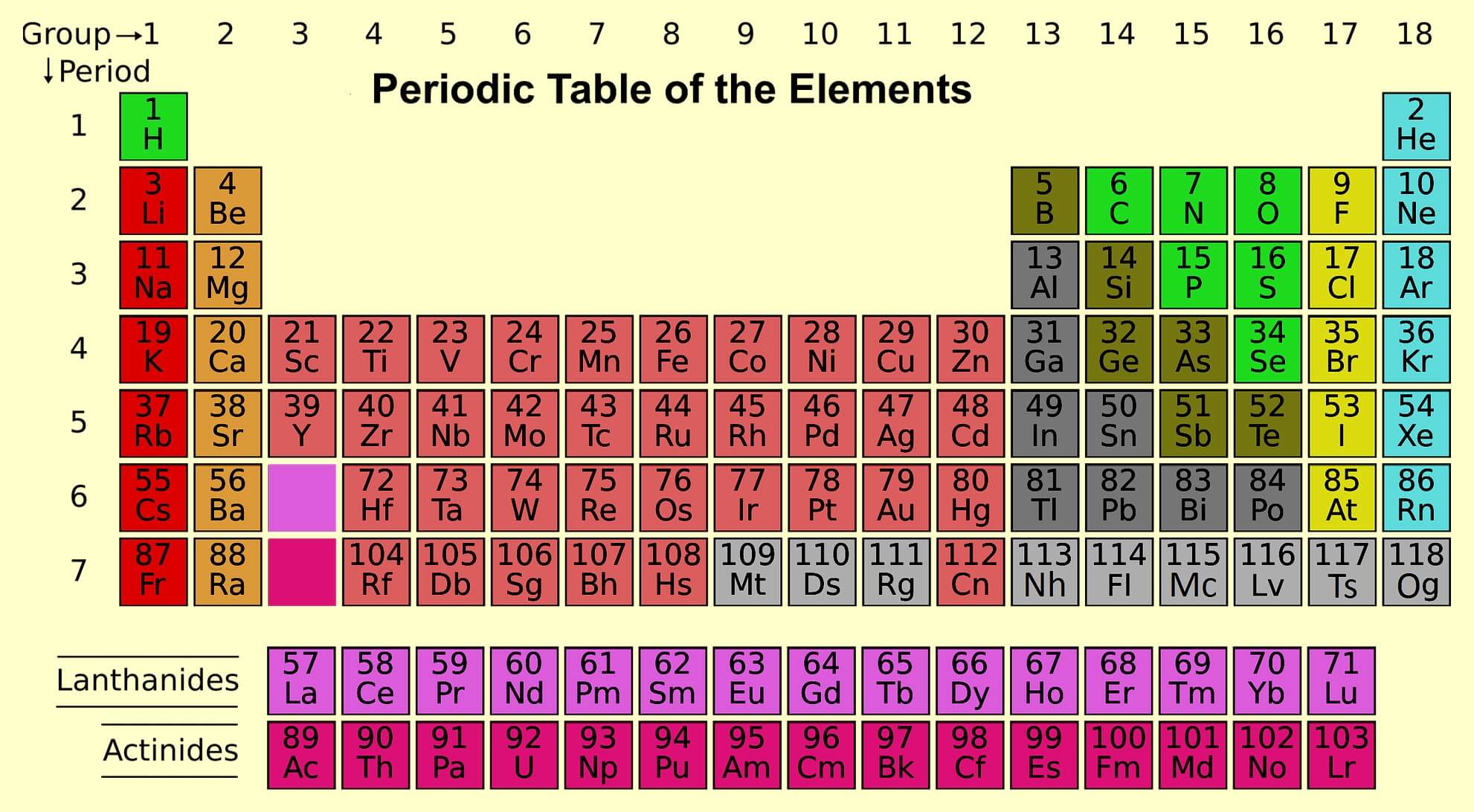
The long form of the periodic table is based on the organization of the elements in order of their atomic number, which is the number of protons in the nucleus of an atom. This organization is based on the fundamental principles of atomic structure, which dictate the properties and behaviors of the elements.
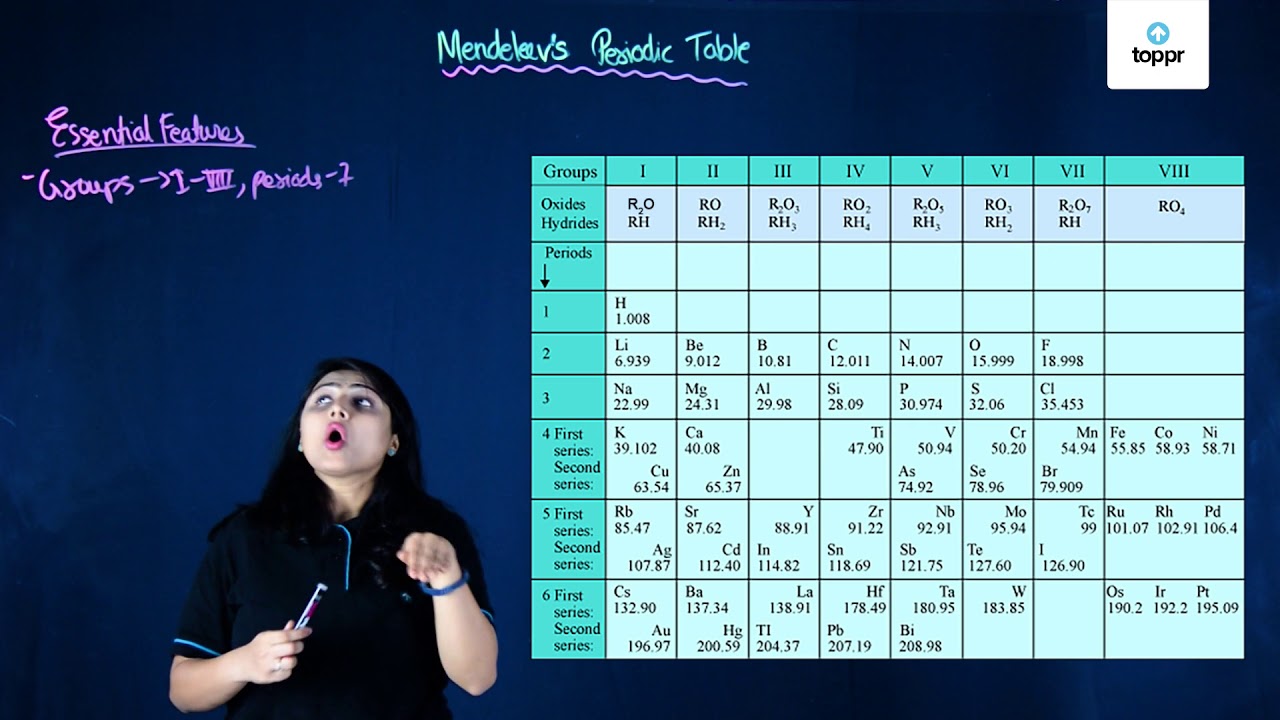
The periodic table was first proposed by Russian chemist Dmitri Mendeleev in 1869. He arranged the elements into a table based on their atomic mass, with similar properties placed in columns, which he called groups. However, it was later discovered that the atomic mass did not accurately reflect the properties of the elements, and the modern periodic table is based on atomic number rather than atomic mass.
The long form of the periodic table includes all of the known elements, arranged in order of increasing atomic number. It is divided into 18 vertical columns, called groups, and seven horizontal rows, called periods. The elements in each group have similar chemical properties, and the elements in each period have similar physical and chemical properties.
The long form of the periodic table also includes several subcategories that further classify the elements. For example, the elements in the main group elements, also known as the representative elements, are found in the s- and p-blocks of the periodic table. These elements include the alkali metals, alkaline earth metals, halogens, and noble gases.
The transition elements, also known as the d-block elements, are found in the middle of the periodic table. These elements have intermediate electronegativities and are known for their ability to form complex ions. The inner transition elements, also known as the f-block elements, are found at the bottom of the periodic table and have unique electron configurations.
The long form of the periodic table is an essential tool for chemists and scientists in predicting the properties and behaviors of the elements. It helps to understand the relationships between the elements and their role in the world around us. The periodic table continues to evolve as new elements are discovered and our understanding of atomic structure deepens.